Figure 2.
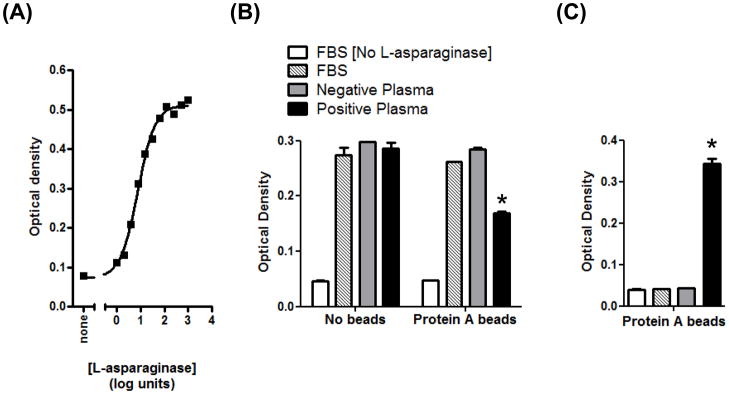
Canine anti-asparaginase antibodies immunoprecipitate, but do not directly inhibit, enzyme function. (A) Titration of enzyme activity. Dilutions of L-asparaginase were incubated with the substrate AHA at 37°C, and hydrolysis was measured as indooxine production, as described in the Materials and Methods. The EC50 is 7.5 units mL−1 (95% confidence intervals are 5.5–10.3). The data represent two independent repetitions of the experiment. (B) Positive plasma binds soluble L-asparaginase, and subsequent removal of total IgG with protein A reduces asparaginase activity in the supernatant. Plasma from ELISA-positive and –negative dogs, or FBS control, were incubated with L-asparaginase overnight, prior to the addition of a protein A agarose bead slurry, or PBS, for 3 h. The final concentration of L-asparaginase was 6 units mL−1. When all treatments containing L-asparaginase were tested against the control sample (FBS without beads), only the activity in the starred group was significantly decreased (P<0.001). (C). Soluble L-asparaginase activity removed by immunoprecipitation with plasma and protein A is retained on the agarose beads. Beads from (B) were washed five times with PBS, and then incubated with the AHA substrate. Only the starred group possessed significant enzyme activity (P<0.001) when compared to the control lacking L-asparaginase.
