Figure 3.
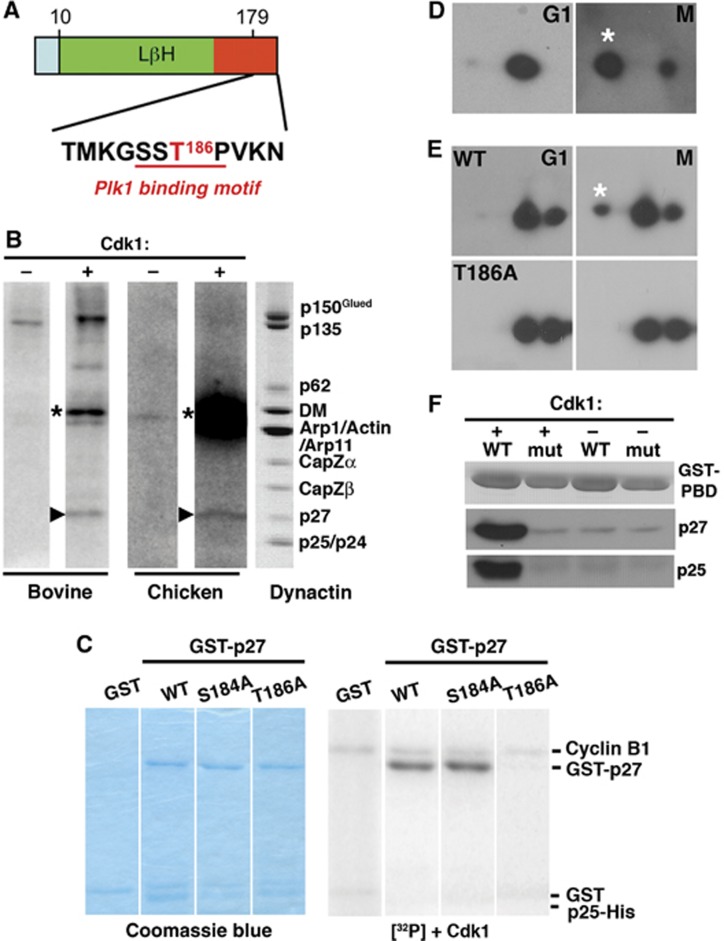
p27 phosphorylation and Plk1 binding. (A) Location in mammalian p27 of the predicted Cdk1 phosphorylation site (T186, red; Group-based Prediction System; GPS Version 2.1, http://gps.biocuckoo.org/) and the Plk1 binding motif (red underline). (B) In vitro phosphorylation of native bovine and chick embryo brain dynactin. The autoradiograms (left four lanes) show samples incubated in the absence (−) or presence (+) of purified Cdk1. Arrowheads indicate p27; asterisks mark cyclin B, which is autophosphorylated. The lane on the right shows bovine dynactin (Coomassie blue stain). (C) In vitro phosphorylation by Cdk1 of bacterially expressed GST-p27/p25His6 complexes (wild-type p27 and the T186A and S184A variants) and a GST control. Left: Coomassie blue-stained gels, right: the corresponding autoradiograms. (D) In vivo phosphorylation of endogenous p27. Lysates prepared from Cos7 cells synchronized in G1 (17 h after double thymidine block) or M (via nocodazole arrest and shake off) were subjected to two-dimensional immunoblotting for p27. (E) In vivo phosphorylation of exogenously expressed wild-type and T186A EGFP-p27 was evaluated as in (D). (F) In vitro binding of purified p27/p25 heterodimers to GST-tagged wild type PBD (wt) and a non-binding variant (mut) (Elia et al, 2003). Proteins immobilized on glutathione beads were incubated with purified p27/p25 heterodimers that had been phosphorylated in vitro using purified Cdk1. Phosphorylation was verified by ProQ staining. +: phosphorylated p27; −: unphosphorylated p27.
Source data for this figure is available on the online supplementary information page.