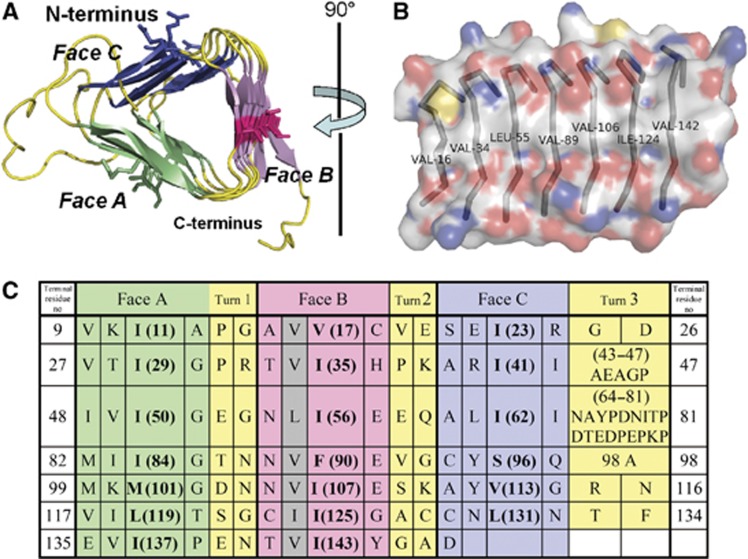
Figure 4.
The structure of the LβH domain of human p27 (PDB accession number: 3TV0). (A) A view of the crystallographic model of the LβH domain looking down the main axis of the β-helix, from the N-terminus towards the C-terminus. The unique features of the β-helical fold, and residues in the key position 2, are shown in detail using different colours. (B) A view looking directly at Face B of the LβH. The molecular surface is coloured according to element (C: white, N: blue, O: red, and S: yellow). (C) Structure-based amino-acid sequence alignment of the seven ‘rungs’ of the p27 β-helix, coloured to match the structural model in (A). Amino acids that face the interior of the β-helix and thus contribute to the hydrophobic core are indicated in bold. The grey shading indicates the hydrophobic amino acids in Face B (the central ridge in B) that may contribute to the dimer interface.