Abstract
Multiple sclerosis (MS) is a disease characterized by inflammation and demyelination. Currently, the cause of MS is unknown. Experimental autoimmune encephalomyelitis (EAE) is the most common mouse model of MS. Treatments with the sex hormones, estrogens and androgens, are capable of offering disease protection during EAE and are currently being used in clinical trials of MS. Beyond endogenous estrogens and androgens, treatments with selective estrogen receptor modulators (SERMs) for estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) are also capable of providing disease protection. This protection includes, but is not limited to, prevention of clinical disease, reduction of CNS inflammation, protection against demyelination, and protection against axonal loss. In EAE, current efforts are focused on using conditional cell specific knockouts of sex hormone receptors to identify the in vivo targets of these estrogens and androgens as well as downstream molecules responsible for disease protection.
Keywords: EAE, MS, ERα, ERβ, Estriol, Estradiol, Testosterone, 5αDHT, Neuroprotection
1. Multiple sclerosis (MS)
Multiple sclerosis (MS) is an autoimmune disease characterized by inflammation and demyelination in the CNS from unknown causes [77,143]. Clinical symptoms usually begin to occur in young adults. Nearly 80% of patients develop a relapse-remitting (RR-MS) course of disease, which can eventually become a more chronic secondary progressive (SP-MS) form after many years. About 15% of patients exhibit disease progression from the start called primary progressive MS (PP-MS) [21]. With myelin thought to be the primary target of the immune cells, areas of inflammation and demyelination constitute the MS lesions, with these lesions classically in white matter [65]. Lesions have been further subdivided into categories of active, chronic active and chronic inactive based on pathology [64,74]. Axonal transection and axonal loss can occur in lesioned areas perhaps contributing to permanent disability. Pathology also exists in normal appearing white and gray matter as demonstrated by magnetic resonance imaging (MRI) and pathologic studies of post-mortem MS tissue [54].
MS is classically viewed as a CD4+ Th1 mediated immune disease. However, a host of immune and non-hematopoietic cells are also involved in the course of the disease including CD8+ T-cells, B-cells, macrophages, dendritic cells, astrocytes and oligodendrocytes to name a few [77,85,132]. One major question in MS involves understanding whether immune inflammation is a primary cause or a secondary effect of neurodegeneration [142]. Current MS treatments only treat the inflammatory component of the disease. However, neurodegeneration continues to occur even when inflammation is suppressed. Thus, while neurodegeneration may be triggered initially by inflammation, it appears that a neurodegenerative process may continue and eventually become relatively independent of inflammation. Therefore, there is a need for a neuroprotective treatment in combination with an anti-inflammatory treatment to potentially achieve complete protection during MS [86,120].
2. Experimental autoimmune encephalomyelitis (EAE)
EAE is the most common mouse model of MS [24,49]. It is a CD4+ Th1/Th17 mediated autoimmune disease in which perivascular T-cells, followed by macrophages, enter the CNS, leading to lesioned areas of demyelination and axonal loss [54]. This demyelination and axonal loss correlates with motor deficits in standard EAE clinical scores which primarily assess walking ability [152]. EAE has strain specific effects. In the SJL model, the disease is relapse-remitting, similar to that of RR-MS. In the C57BL/6 model, EAE follows a more progressive course, resembling PP-MS or SP-MS [24]. EAE may be either active or adoptive depending upon the method of induction [87]. The basic concept of both active and adoptive EAE is to induce an immune response to a myelin antigen. In active EAE, an animal is immunized with a myelin antigen along with the nonspecific immune stimulators complete Freund’s adjuvant (CFA) and tuberculosis bacterium (TB). Pertussis toxin (PTx) is also given in active EAE. The T-cells generated through this immunization regimen are able to cross the blood to brain barrier (BBB) and propagate an influx of monocytes into the CNS. They then alter the cytokine/chemokine profile of the cells there in. Subsequent activation of resident microglia, and perhaps CNS cells such as astrocytes, simultaneously occurs as well. All of these steps lead to the demyelination of axons and axonal transection both within and beyond these areas of immune cell infiltration. Thus in active EAE, both the induction of the immune response and the neurodegenerative effects of the immune attack occur in the same mouse. In contrast, adoptive EAE separates the induction and the effector phase of EAE so that each phase can be studied separately. The induction phase is started in a donor mouse, in which the immunization is given over draining lymph nodes. The lymph nodes cells (LNCs) are then harvested and re-stimulated ex-vivo with the myelin antigen. These cultured T-cells and monocytes are then injected into a recipient animal to begin the effector phase of adoptive EAE. Immune infiltration then occurs in the CNS, followed by demyelination and axonal loss in the recipient mouse [87]. Thus, the central difference between active and adoptive EAE is that in adoptive EAE the initiation of the immune response occurs in a different mouse, the donor, than the one who exhibits disease, the recipient. This separation of immunopathology phases in adoptive EAE allows researchers to tease apart effects in the immune response from those in the CNS. For example, in adoptive EAE, one could treat a donor mouse with a compound, such as a hormone, and then inject the donor mouse’s LNCs into an untreated recipient to understand how the hormone treated LNC’s might differ in their ability to induce disease in the CNS of a recipient mouse. While the EAE model has its limitations, it has been the major preclinical model used to derive many current MS treatments [24,89,129].
3. Gender differences in MS and EAE
There are large gender differences in the prevalence of human autoimmune diseases. Most of these gender differences entail a higher incidence in females as compared to males: systemic lupus erythematosis (SLE), rheumatoid arthritis (RA), Graves disease, and multiple sclerosis (MS) [9,37,73,78,84,98,103,125]. While the reasons for these gender differences are still unknown, possible explanations include differences in sex hormones or sex chromosomes. Specifically, possibilities include: (1) a detrimental effect of endogenous ovarian hormones such as estrogens and/or progesterone in females, (2) a protective effect of endogenous testicular hormones such as androgens in males, or (3) a detrimental effect of the XX sex chromosome compliment in the females, or (4) a protective effect of the XY sex chromosome compliment in males [93,138,147]. The EAE model has been useful to study these four non-mutually exclusive possibilities.
Similar to MS, sex differences exist in the EAE model. In the SJL, ASW, and NZW strains, female mice are more susceptible to EAE than males. However, in the B10.PL and PL/J strains, male mice are more susceptible to EAE than females [107]. Ovariectomy of SJL female mice is controversial, with some groups showing no effect on disease and others showing some worsening of EAE scores [56,83,148]. Together, these results suggest that endogenous estrogens are clearly not detrimental to EAE in females, but if anything may be protective. On the male side, castration of mice worsens EAE in the SJL strain, suggesting that endogenous androgens are indeed protective to males [5,148]. It is important to keep in mind that while most sex hormones come from the gonads, the CNS, particularly during EAE, is capable of providing endogenous estrogens and androgens [19]. However, whether these levels reach physiological relevance remains unclear.
Differences in sex chromosomes also may contribute to sex differences in disease. By using mice which differed in sex chromosome complement while sharing a common gonadal phenotype [4] it has been shown in the SJL strain that the XX sex chromosome compliment confers increased severity of EAE as compared to the XY sex chromosome compliment. This suggested that either the XX complement is disease promoting or the XY complement is protective [106,130]. Other studies have shown that a Y chromosome-linked polymorphism can influence disease [139]. Specifically, EAE was more severe in young male consomic mice carrying a B10.S Y chromosome as compared to age and sex matched SJL mice. The authors therefore concluded that a polymorphismon the Y chromosome of SJL mice contributes to the well known observation that young male mice exhibit temporary protection from disease [133]. It remains unclear as to why a polymorphism would be protective in younger, but not older, mice. Together the data would suggest an interaction between sex chromosome genes and other factors related to aging, with these other factors theoretically being either hormonal or nonhormonal. One possible nonhormonal factor, regardless of age, could be genomic imprinting. Previous research has suggested that genomic imprinting such as histone modifications, methylation, and endogenous miRNA could possibly play a role in autoimmunity and sex differences [18,38]. In summary, while many assume that the sex bias in MS is due to sex hormones, one must appreciate that sex chromosome genes may also play a role.
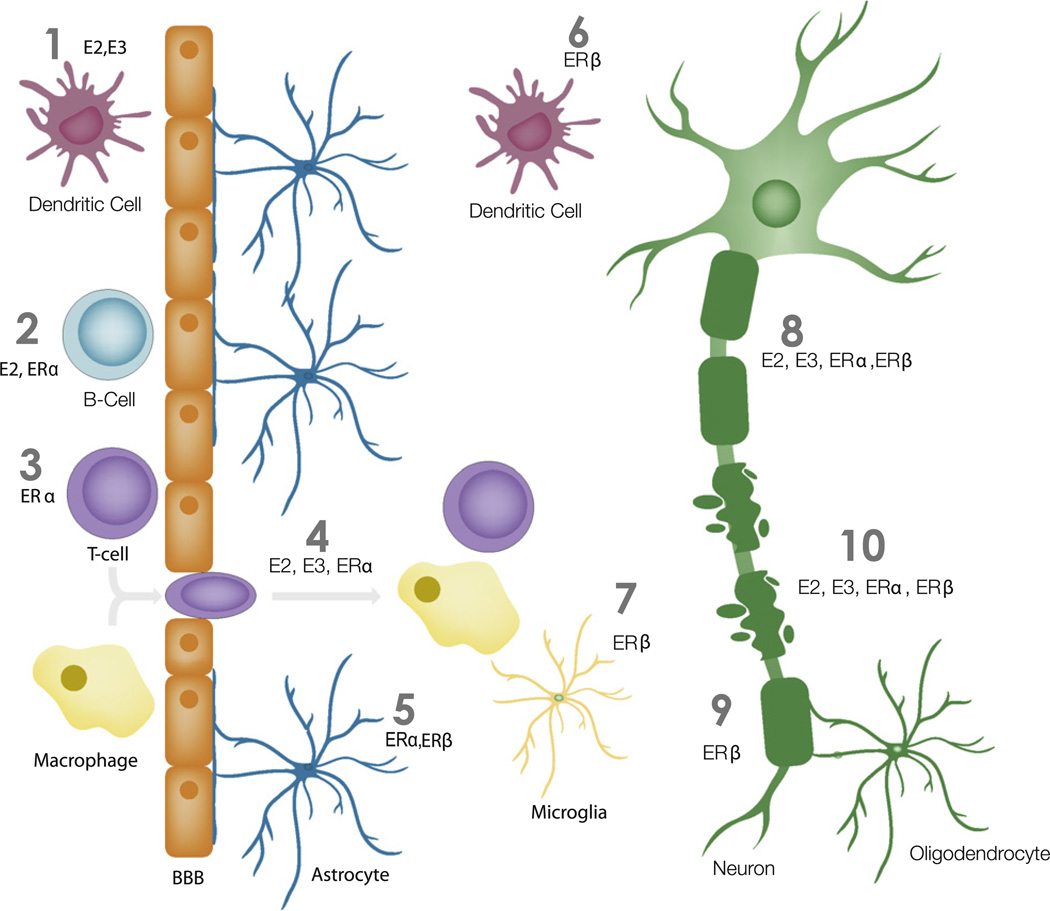
Currently, there is an extensive amount of literature highlighting the effect of estrogens and androgens in EAE and MS [34,48,99,100, 148]. In this review we will build upon this existing literature by focusing on specific sex hormone receptors and how they contribute to disease protection during EAE and MS (see Fig. 1).
Fig. 1.
Simplified model of estrogen’s actions for disease protection during EAE. (1) Estradiol (E2) and estriol (E3) act on dendritic cells (red) in the periphery to provide protection during EAE [71,108]. (2) Estradiol and ERα ligand act on B-cells (light blue) to provide protection during EAE [11]. (3) ERα ligand acts on T-cells (purple) to prevent EAE [67]. (4) Estradiol, estriol, and ERα ligand prevent inflammation into the CNS during EAE [7,59,66,92,141]. (5) ERα and ERβ act on the astrocytes (dark blue) to promote disease protection [122,134]. (6) ERβ acts on dendritic cells (red) in the CNS to provide protection during EAE [33]. (7) ERβ acts on microglia to provide protection during EAE [122]. (8) Estradiol, estriol, ERα and ERβ ligand treatment during EAE prevent axonal loss [7,59,92,141]. (9) ERβ ligand promotes remyelination of axons during EAE [23]. (10) Estradiol, estriol, ERα and ERβ ligand prevent demyelination during EAE [7,59,92,141].
4. Pregnancy in MS and EAE
In MS, during the last trimester of pregnancy, circulating levels of estrogens are at their highest levels correlating with a reduction in relapse rates among women with MS. Post-partum levels of estrogens drop precipitously and correlate with a significant increase in relapse rates during the 3–6 months after delivery [22]. While controversial, some reports have demonstrated that pregnancy could offer long term protection to women with MS [121,146]. MS patients who had one or more pregnancies were wheelchair dependent after 18.6 years, versus 12.5 years for the control group [146]. Others have shown that pregnancy can regulate the immune response in MS patients. Peripheral blood lymphocytes from pregnant women with MS were mitogen stimulated to examine the Th1 and Th2 cytokine profile during pregnancy and post-partum. The patients showed a remarkable shift from Th2 profile during pregnancy to a Th1 cytokine profile post-partum, further providing evidence of pregnancy’s effect in this Th1 mediated disease [2].
Pregnancy also offers protection to female animal models with EAE [15,36,43,61,83]. Specifically, pregnant SJL and C57BL/6 mice showed a disease amelioration compared to non-pregnant controls. Further, lymphocytes from pregnant EAE mice showed a decrease in TNFα and IL-17 with an increase in IL-10 [83]. Given the powerful effect of late pregnancy on EAE and MS, and since estrogens are at high levels during late pregnancy, studies have examined if exogenous estrogens are capable of providing disease protection in EAE and MS.
5. Estrogens and neuroprotection
Estrogens are made up of three endogenous biologically different compounds: estrone, estradiol, and estriol [44,157]. Furthermore, exogenous selective estrogen receptor modulators (SERMs) are capable of activating either estrogen receptor alpha (ERα) or estrogen receptor beta (ERβ) on select tissues [68]. Testosterone and dihydrotestosterone (DHT) are able to act on the androgen receptor (AR). However, DHT is also able to act indirectly through ERβ [63,75]. It is always difficult to decipher the action of testosterone since it can be aromatized to estrogens through the enzyme aromatase. Hence, either the non-aromatizable 5αDHT, or AR antagonists can be used to study the effect of androgens on actions of ARs [44,57].
Treatments with estrogens have demonstrated diverse mechanisms of possible neuroprotection in a number of diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AZ), ischemic stroke, spinal cord injury and MS [14,20,40,48,123,137]. In clinical studies, women who took estrogen replacement therapy were at less risk of developing PD [25]. Woman who received short-term estrogen treatment had an increase in dopamine transporters in the caudate putamen [41]. Multiple groups have shown that in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) animal model of PD, exogenous physiological levels of estradiol were able to protect against loss of DA neurons [58,117,126]. Others have shown that the in PD animal model, 6-hydroxdopamine (6-OHDA), estradiol can also act indirectly by activating the insulin-like growth factor-1 (IGF-1) receptor to protect against 6-OHDA induced neuronal loss [116].
In AD patients, estrogen treatment decreased the risk of disease, particularly in younger (50–62yo) patients, but was not effective in older groups of patients [53]. This early protection during AD could be due to estradiol’s ability to increase amyloid beta-protein (Aβ) uptake by microglia as well as prevent Aβ peptide formation by neurons [69,153]. It is known that in spinal cord injury, estrogen can protect against neuronal loss. Data suggest that this prevention of neuronal loss could be due to estradiol’s ability to prevent the actions of Ca2+ activated proteases [118,123]. Furthermore, others have shown that estradiol was able to reduce neuronal death and loss of lesion volume in ischemic stroke [46,137].
Estrogens can also act on other cells in the diseased CNS besides neurons. For instance, estrogen increases astrocytes ability to uptake glutamate to prevent neuronal loss due to glutamate toxicity [3,29]. Others have shown that estrogen can increase transforming growth factor beta (TGFβ-1) and TGFβ-2 from astrocytes [30]. Estrogens also have a suppressing effect on neuroinflammation [145]. Estradiol strongly inhibits microglia activation by lipopolysaccharide (LPS) [31,144]. In addition, estradiol can inhibit intracellular localization of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB), which can prevent downstream activation of a variety of inflammatory genes [45].
With estrogen’s ability to provide protection in a number of CNS disease models, much research has been focused on the mitochondria as a target of estrogens actions. Treatments with estrogens have a direct neuroprotective effect on mitochondria [16,55,95,96,97]. Estrogens have been shown to increase aerobic glycolysis, respiratory efficiency, ATP generation, Ca2+ load tolerance and act as an antioxidant defense [17]. Furthermore, estrogens can alter pro and anti-apoptotic molecules that act on the mitochondria. For instance, estrogen can increase anti-apoptotic markers, bcl-2 and bcl-xl, while decreasing the pro-apoptotic marker bax in animal models of AD [96]. Estrogen’s antioxidant properties also allow it to protect against reactive oxygen species from the mitochondria [119]. It is important to note that the timing of estrogen administration is critical to ensure estrogens protective outcome as noted in the healthy cell bias of estrogen action hypothesis. If estrogens are administered too late in the disease they are not protective [17,94,124,156].
6. Estradiol and EAE
Treatment with a wide dosage range of estradiol is able to ameliorate EAE during both the induction and effector phases of both active and adoptive EAE. This amelioration can occur in both sexes of mice, as well as multiple mouse strains with EAE [7,35,56,79,95,135,148]. The protective effects of estradiol in EAE include a decrease in inflammatory cells in the CNS as well as protection against demyelination [7,99,135]. With respect to inflammation, estradiol treatment was able to alter cytokine production as well as alter chemokine/chemokine receptors, growth factors, and adhesion molecules in EAE mice. Specifically, estradiol treatment was able to decrease chemokine (C–C motif) ligands and receptors, tumor necrosis factor-alpha (TNF-α), and others, while increasing transforming growth factor beta (TGFβ)-2 and TGFβ-3 mRNA in the spinal cord of EAE mice [79,81,135] (Table 1). Further studies have shown that mononuclear cells isolated from the brain of estradiol treated EAE mice show a decrease in TNFα, IFNγ, and others [11,135] (Table 1). In splenocytes and/or peripheral LNCs, estradiol was able to decrease TNFα, neural cell adhesion molecule (NCAM) and others while increasing cytotoxic T-lymphocyte antigen-4 (CTLA-4), vascular cell adhesion molecule (VCAM) and more [11,67,79,80,135] (Table 1).
Table 1.
Treatment with exogenous estrogens and androgens provide immune protection in EAE and MS in multiple tissues/cells. Select cytokines/chemokines and their respective receptors as well as adhesion molecules, growth factors and proteinases are subject to increase or decrease with treatment of ERα ligand, ERβ ligand, estradiol (E2), estriol (E3), testosterone (T), and 5aDHT in splenocytes, lymph nodes, dendritic cells and CNS in EAE as well as PBMCs in MS patients. CNS includes mononuclear cells isolated from the CNS. Arrows indicate whether the change was an increase or decrease with the sex hormone treatment. Note: Some of the names of the molecules have been changed from their original name to reflect current nomenclature.
| EAE Peripheral splenocytes/LNCs |
EAE Dendritic cells |
EAE CNS |
MS PBMCs |
|
|---|---|---|---|---|
| ERα ligand | ↑IL-5 [92,141] ↓TNFα, IFNγ, IL-2, IL-4, IL-6, IL-10, MMP9 [35,51,92] |
N/A | N/A | N/A |
| Erβ ligand | ↓IL-17 [32] | ↓TNFα [22] | N/A | N/A |
| Estradiol | ↑CCL2, CCL4, IL-18, TFGβ-3, CTLA-4, CCR3, VCAM [11,71,81,135] ↓ TNFα, IL-2, IFNγ, IL-6, IL-17, CCL2, CCL5, CCR1, CCR5, NCAM [11,71,81,135] |
↓TNFα, IFNγ, and IL-12 [71] | ↑TGFβ-2, TGFβ-3, IL-1β, IL-17, CCL2, CCL5, CXCL13 [11,79,81,135] | N/A |
| Estriol | ↑IL-5, IL-10, Il-4 [59,104,108] ↓TNFα, IFNγ, IL-2, IL-6, MMP-9 [51,59,104,108] |
↑TGF-β, IL-10, IL-12p35 [108] ↓IL-12p40, IL-23p19, IL-6 [108] |
N/A | ↑IL-5, IL-10[127,131] ↓TNFα, IFNγ, MMP-9 [127,131] |
| Testosterone/DHT | ↑IL-10 [6,26] ↓IFNγ, TNFα [6,82] |
N/A | N/A | ↑BDNF, PDGFBB, TGFβ1 [50] ↓IL-2 [50] |
Dendritic cells appear to be one potential target of estradiol’s protective actions during EAE. Dendritic cells are potent antigen presenting cells and are thought to play a role in T-cell activation in EAE [1]. Estradiol treatment resulted in a decrease in activated dendritic cells in the CNS and spleens of EAE mice. Mature dendritic cells were shown to have decreased expression of TNFα, IFNγ, and IL-12 mRNA with estradiol treatment (Table 1). Finally, T-cells cocultured with dendritic cells that were pre-treated with estradiol showed a shift from Th1 to Th2 cytokine production [71]. Further research has also shown that dendritic cells are a target of estradiol in Lewis rats with EAE. Rats injected with dendritic cells that were pre-treated with estradiol showed a decrease in CD4+ T-cells and CD68+ macrophages in the CNS compared to naïe injected dendritic cells [110].
Others have focused their research on T-cells as the target cell of estradiol’s protective effect [102,101,113]. Studies have shown that the decrease in T-cells into the CNS of estradiol treated mice was not due to a decrease in T-cell proliferation, but in fact, the ability of T-cells to enter the CNS [7,135]. Although estradiol might not directly act on T-cells during EAE to provide disease protection, estradiol appears to indirectly alter the sub-populations of T-cells [101]. For example, matrix metallopeptidase-9 (MMP-9), has been shown to allow T-cells to migrate across the BBB. Research has shown that estradiol was able to decrease MMP-9 expression on T-cells during EAE [51], perhaps explaining why T-cell numbers are decreased in the CNS of estradiol treated EAE mice [135]. Furthermore, estradiol was able to upregulate FoxP3, contributing to a protective effect from CD4+ CD25+ regulatory T-cells [99,100,114]. However, removal of Foxp3+ T-cells did not appear to alter estradiol’s protective effect in EAE mice, suggesting other cells, such as B-cells or CNS cells might be responsible for estradiol’s protection [136].
B-cells also appear to be indispensible in responding to estradiol’s protective effects during EAE [136]. Removal of B-cells from EAE mice abrogates the protective effects of estradiol [11]. When the B-cells were examined from estradiol treated mice, there was an upregulation of programmed cell death ligand-1 (PD-L1) as well as an increase in IL-10 [11]. Further research is needed to fully understand estradiol’s actions on B-cells in EAE.
7. Estrogen receptor alpha ligand and EAE
Given that estradiol is able to act both on ERα and ERβ, a host of research has now been focused on which receptor might be responsible for the protective effects of estrogens during EAE. While data have shown that this effect is mediated through ERα [66,67,111], other data have shown that ERβ can also offer protection during EAE [23,33].
Originally it was shown that EAE was less severe in B10.PL male mice with a global knockout of the ERα gene, termed Esr1 (−/−), as compared to WT, Esr1 (+/+) male mice, suggesting that endogenous estrogens are disease promoting during EAE [112]. The source of estrogens in these male mice was unclear, but in retrospect it could have been from conversion of testostosterone to estradiol in vivo by aromatase or from injury induced upregulation of estradiol production locally within the CNS. Later data from the same group, as well as another, each treated wild type and global ERα knock out mice with higher than peak ovulatory doses of either estradiol or estriol, respectively, to ascertain whether these exogenously administered estrogens worked through ERα to ameliorate EAE [72,111]. While these two papers each showed a critical role for ERα in disease protection mediated by exogenous estradiol or estriol treatment, there were no differences in disease between placebo treated global ERα knock outs and wild types in either females or males. Notably, as discussed elsewhere, the effect of ovariectomy on EAE has been controversial, with some groups finding no significant consistent effect of ovariectomy on EAE [148] and others finding a worsening of EAE thereby suggesting a disease protective effect of endogenous estrogens [56]. Together, the lack of an effect of the global ERα knock out on placebo treated mice with EAE is consistent with the lack of an effect of ovariectomy on disease.
Exogenous ERα ligand, propyl pyrazole triol (PPT), treatment provides disease protection in a variety of mouse strains with EAE [35,51,92,134,141]. In active EAE, pre-treatment with the ERα ligand abolishes clinical disease as evidenced by the loss of this protection in different ERα KO mouse models [92,111]. Furthermore, in the effector phase of EAE, ERα ligand treatment provides clinical disease protection compared to EAE with vehicle control treatment. Although the disease was not completely abolished with treatment only in the effector phase, the peak disease score was lower and the overall severity of the disease was significantly reduced [134].
In the peripheral immune system during active EAE, ERα ligand treatment was able to decrease pro-inflammatory cytokines such as TNFα, IFNγ, and more while increasing the anti-inflammatory cytokine IL-5 in cultured splenocytes and LNCs [35,92,141] (Table 1). Within the CNS, ERα ligand treatment was capable of decreasing macrophage and T-cell inflammation at both early and late time points of EAE [92,141]. Beyond inflammation, ERα ligand treatment has been shown to protect against axonal loss as well as demyelination at both early and late time points of EAE [92,141]. While the mechanism of this protection is not fully understood, recent studies have shown that ERα ligand treated mice with EAE had reduced MMP-9 in supernatants from auto-antigen stimulated splenocytes. This reduction in MMP-9 coincided with a decrease in T-cell and macrophage infiltration into the CNS [51] (Table 1).
Given the powerful effects of ERα ligand treatment on EAE, focus shifted toward what cell or host of cells were responsible for ERα ligand’s protective actions. ERα is expressed on a variety of cells including but not limited to: T-cells, macrophages, microglia, neurons, oligodendrocytes, astrocytes, and epithelial cells [27,39,42,52,88,109,144]. It was assumed that ERα ligand was acting on peripheral immune cells to prevent an immune response in EAE, thus ameliorating disease. However, bone marrow chimera studies and selective ERα KO studies, in which ERα was selectively removed from T and/or B-cells, demonstrated that peripheral T and B-cells were not the direct target cell of estrogen mediated protection [42]. However, a recent study used bone marrow chimeras to generate a mouse that was deficient in ERα on hematopoietic cells. While there was no effect on the clinical course of EAE, the mice that lacked ERα on hematopoietic cells had a larger amount of CD4+ T-cell infiltration into the CNS, suggesting that ERα expression on immune cells was involved in reducing CNS infiltration [66]. A new study has now shown that T-cells may indeed be a direct target of ERα ligand’s protective effects depending upon the timing and dosage of exogenous ERα ligand treatment [67]. Furthermore, B-cells also appear to be a target cell of interest. Estradiol treated B-cells that were removed from ERα −/− mice were unable to suppress a proliferative response from co-cultured T-cells when compared to B-cells from WT mice, thus demonstrating that estradiol acted through ERα on B-cells to inhibit an immune response [11]. Clearly, more research is necessary to determine if T and/or B-cells are the direct or indirect targets of ERα ligand treatment in vivo during EAE.
Regarding potential cellular targets in the CNS, astrocytes are known to express ER’s [39]. Removal of reactive astrocytes was shown to worsen EAE. Specifically, clinical disease, as well as levels of CNS inflammation and axonal loss, were increased in EAE animals that had transgenic ablation of reactive astrocytes compared to WT mice with EAE [149]. Thus, a conditional knock out, whereby ERα was removed only in astrocytes, was created. When EAE was induced in this conditional knockout of ERα from astrocytes, ERα ligand treatment was no longer protective. Specifically, ERα ligand treatment of mice that had a conditional knockout of ERα from astrocytes, were no longer protected from either T-cell and macrophage infiltration into the CNS or axonal loss when compared to WT mice. Thus, ERα expression on astrocytes, is indispensable in providing ERα ligand mediated protection [134]. While the exact mechanism of this ERα astrocyte protection is unknown, recent evidence has shown that during EAE, ERα was capable of preventing TNFα induced CCL2 expression in astrocytes, thereby preventing NF-kB transcription in the astrocyte [47]. Given that NF-kB is a powerful pro-inflammatory regulator, perhaps this decrease in transcription was responsible for the inflammatory differences seen in the ERα astrocyte conditional knockout [13,134]. While ERα expression on astrocytes plays an important role in the protective effect of ERα ligand treatment, further research is needed to determine if there are other cellular targets in the CNS that may mediate ERα ligand’s neuroprotective effects.
8. Estrogen receptor beta ligand and EAE
Data have shown that the global knock out of the ERβ gene does not alter EAE disease severity compared to WT mice, suggesting endogenous estrogens do not act through ERβ to offer disease protection [112,111]. However, exogenous treatment with an ERβ ligand has been shown to ameliorate EAE in the C57BL/6 strain, but not in the SJL strain [35,141]. This difference in clinical protection could be due to strain differences or due to differences in the timing of the effect of treatment on disease [32,35,51,141]. Our group has shown that EAE mice pre-treated with ERβ ligand exhibit lower disease scores than mice treated with vehicle. Although the disease was not completely abolished, clinical scores were significantly lower later in disease [141]. In contrast to treatment with ERα ligand, ERβ ligand treatment did not affect cytokine production during peripheral auto-antigen specific immune responses. In addition, ERβ ligand treatment did not reduce spinal cord inflammation during EAE as assessed by immunohistochemistry (IHC) of the spinal cord. Nevertheless, treatment with ERβ ligand was still able to protect mice against demyelination and axonal loss. Further, ERβ ligand treatment was able to restore motor function as measured by the rotor-rod test in EAE mice [141]. These results suggested that ERβ, while clinically beneficial, did not reduce levels of CNS inflammation and therefore might act directly on CNS cells to mediate neuroprotection.
Given ERβ ligand’s potential as a neuroprotective treatment for MS, our group studied the combination of ERβ ligand treatment with an anti-inflammatory agent currently used in MS, Beta-interferon. These studies in EAE confirmed that ERβ ligand treatment alone was effective at reducing clinical disease, as well as protecting against axonal loss even in the presence of inflammation [32]. However, the combination of ERβ ligand treatment with Beta-interferon was more effective at ameliorating EAE than ERβ ligand treatment alone. Combination treatment significantly reduced clinical disease, preserved axonal density, and reduced inflammation. Specifically, combination treatment reduced T-cells and macrophages in the spinal cord, while decreasing IL-17 from splenocytes. In addition, the cell adhesion molecule, VLA4, was significantly reduced on T-cells with the combination treatment [32]. These data suggested that ERβ ligand treatment might be useful in combination with an anti-inflammatory agent in the treatment of MS.
Recent evidence has shown that ERβ ligand treatment was not only capable of preventing demyelination, but also has the ability to stimulate endogenous myelination [23]. In the corpus callosum, similar to previous observations in the spinal cord, ERβ ligand treatment prevented demyelination of callosum axons even in the presence of inflammation [23,141]. Furthermore, there was an increase in the number of mature oligodendrocytes that correlated with an increase in myelin sheath thickness with a corresponding decrease in the g-ratio. Electrophysiology recordings showed that callosum axon conduction, with ERβ ligand treatment, exhibited a significant increase in compound action potential amplitudes, latency, and axon refractoriness [23]. It remains unknown whether effects on myelination are due to direct effects of ERβ ligand on oligodendrocytes or indirect effects on other cells expressing ERβ.
While previous studies showed that ERβ ligand treatment does not affect the levels of inflammation in the CNS [141], subsequent studies asked whether there might be qualitative differences in similar levels of spinal cord inflammation in ERβ ligand versus vehicle treated EAE mice [33]. No differences were observed in the numbers of T-cells or macrophages comprising infiltration, but the numbers of dendritic cells in the CNS were reduced in ERβ ligand treated mice. Furthermore, ERβ ligand treatment decreased the amount of TNFα produced by these CNS dendritic cells (Table 1). Finally, adoptive EAE was induced in WT recipients using draining LNCs composed of dendritic cells from either ERβ KO or WT mice. Upon treatment with ERβ ligand, the immune cells composed of ERβ deficient dendritic cells were no longer sensitive to ERβ ligand mediated immune suppression [33]. Furthermore, others have shown that ERβ ligand treatment can suppress inflammatory responses of microglia and astrocytes [122]. In summary, ERβ ligand differs substantially from ERα ligand in its effects on EAE, but both remain potential candidates for the treatment of MS.
9. GPR30 and EAE
While ERα and ERβ are classically viewed as nuclear receptors, G protein-coupled receptor 30 (GPR30) is expressed on cellular membranes of both human and mouse cells [76,91,115]. Recent evidence suggested that estradiol binding to the GPR30 may contribute to some of the protective effects of estradiol treatment in EAE [10,151,154]. While not lost completely, the magnitude of the estradiol mediated protection during disease, specifically inflammation and demyelination, was reduced in GPR30KO mice on a C57BL/6 background [151,154]. Furthermore, SJL and C57BL/6 mice treated with the GPR30 agonist, G-1, showed a small but significant decrease in clinical disease severity compared to control mice in both active and adoptive EAE [10,151]. G-1 treatment was also able to decrease axonal damage, CNS inflammation, and demyelination [151]. Furthermore, there was a decrease in the number of macrophages in the CNS with G-1 treatment [10]. Cytokine and chemokine expression was also altered with G-1 treatment, specifically there was a decrease in IFNγ, TNFα, IL-17, IL-23 CCL2, CCL4 and CCL5 from cultured splenocytes and LNCs [10]. Additionally, G-1 treatment increased PD-1 in CD4+ Foxp3+ Treg cells, [151]. While G-1 treatment was able to provide some level of protection during EAE, the exact mechanism of action remains unclear.
10. Estriol treatment in EAE
Estriol can bind to both ERα and ERβ, but has a preference for binding ERβ over ERα [63]. Studies have shown that doses of estriol, consistent with physiological murine pregnancy levels, can ameliorate EAE in female mice [7,59]. Estriol treatment was protective in multiple strains of mice including SJL, C57/B6, and B10.Pl. Furthermore, both female and male of SJL and C57BL/6 mice respond to estriol treatment, demonstrating that estriol treatment was not female specific [7,51,56,59,102,104].
Estriol treatment was able to prevent spinal cord inflammation and demyelination in EAE mice [7,59]. Multiple studies have shown that estriol treatment alters the cytokine profile to induce a shift from a Th1 to a Th2 from cultured splenocytes and LNCs. This shift included a decrease in TNFα, IFNγ, IL-2, IL-6, and an increase in IL-5 and IL-10 [7,59,104] (Table 1). Others have shown that splenocytes from estriol treated mice exhibited a decrease in the migration marker MMP-9, consistent with the decrease in CNS inflammation in estriol treated mice [51]. This reduction in MMP-9 was also found with ERα ligand treatment, but not with ERβ ligand treatment [51].
Recent evidence shows that dendritic cells, at least in part, mediate estriol’s protective effect. Estriol treated dendritic cells exhibited a decrease in cytokines: IL-IL-6, IL-12 and more with an increase in the immunoregulatory cytokines: TGF-β and IL-10 [108] (Table 1). Furthermore, activation markers, MHC II, CD80 and CD86, as well as inhibitory markers, PD-L1, PD-L2, B7-H3, and B7-H4 were all increased in estriol treated dendritic cells [108]. These estriol induced changes resulted in a decreased severity of EAE [108]. This evidence in the preclinical model lends support to exploration of estriol as a potential treatment for MS patients.
11. Estriol treatment in MS
A pilot clinical trial of estriol was conducted in females with relapsing-remitting MS (RRMS). In the study, patients were observed for 6 months, then treated with pregnancy levels of oral estriol (8 mg per day) for the following 6 months. After 6 months of treatment the patients were taken off estriol for 6 months and then placed back on treatment for another 4 months. During treatment, RRMS patients exhibited a significant decrease in the number of gadolinium enhancing lesions on brain MRI as compared to pre-treatment baseline. When treatment stopped, enhancing lesions returned to baseline levels, and when treatment was again reinstated, enhancing lesions again decreased. Furthermore, cognitive function, using the paced auditory serial addition test (PASAT), was improved in RRMS patients, although this may have been confounded by practice effects in this single arm cross over study [127].
Estriol treatment was also able to beneficially alter the immune response in MS patients. For example, a decrease in the recall response to a control antigen, namely the delayed type hypersensitivity (DHT) response to tetanus, was observed [127]. Furthermore, during the trial, PBMCS were collected longitudinally and then stimulated ex vivo with mitogens and recall Ags. PBMCs collected during the in vivo estriol treatment period showed a significant increase in IL-5 and IL-10, with a significant decrease in TNFα, IFNγ, and MMP-9 [51,127,131] (Table 1). This Th1 to Th2 cytokine shift correlated with the decrease in enhancing lesions in RRMS patients. Data indicated that the increase in IL-5 was due to an increase in CD4+ and CD8+ T-cells, while the increase in IL-10 was due to an increase CD64+ monocytes/macrophages. The decrease in TNFα was due to a decrease in CD8+ T-cells [131]. Further exploration of estriol as a treatment in MS is warranted and ongoing (http://www.clinicaltri als.gov/ct2/show/NCT00451204).
12. Tamoxifen, raloxifene, and genistein in EAE
Given the possibility of estrogen treatment for MS, studies have explored the use of the SERMs, tamoxifen and raloxifene, and the phytoestrogen, genistein, in EAE [8,28,35]. At a low dosage, both tamoxifen and raloxifene have been shown to reduce the clinical severity of EAE compared to placebo treatment. However, when both estradiol and tamoxifen were used at an equal dose (2.5 mg), estradiol was able to abrogate disease entirely, while tamoxifen only partially reduced disease severity compared to placebo treated mice. Tamoxifen, but not raloxifene, was able to alter the cytokine profile of EAE mice to a Th2 bias. Interestingly both, tamoxifen and raloxifene treatment were able to inhibit CD4+ T-cell proliferation in EAE mice [8,35]. The phytoestrogen, genistein, was also able to reduce clinical disease severity when compared to a placebo control. In addition, genistein treatment increased IL-10, and decreased IFNγ, TNFα and IL-12 [28]. While these three compounds remain possible treatments for MS, much more research is needed before conclusions can be drawn. Specifically, whether these compounds are acting as an agonist for one ER versus an antagonist for another ER in different tissues remains unclear [70].
13. Testosterone, 5αDHT, and EAE
SJL mice show a sex bias similar to humans with MS characterized by greater susceptibility in females as compared to males [26]. It appears that this sex bias is due at least in part to a protective effect of androgens. Castrated SJL male mice show a worsening of clinical disease as well as greater demyelination and inflammation in the spinal cord when compared to sham operated males [5,105]. Interestingly, C57BL/6 mice show neither a sex difference in EAE nor an effect from male castration, suggesting that endogenous androgens are not protective in the C57BL/6 strain. Strangely, this effect is not due to strain differences in endogenous levels of testosterone. In contrast to endogenous testosterone, exogenous testosterone treatment ameliorated EAE in both the SJL and C57Bl/6 strains [6,105]. Interestingly, supplemental testosterone did not provide disease protection in castrated middle-aged male C57BL/6 mice [82]. It remains unclear regarding why endogenous testosterone is effective in the SJL strain but not the C57BL/6 strain; furthermore, it’s unclear why exogenous testosterone is more beneficial in younger mice. Theoretically these differences in the effectiveness of testosterone on disease could be due to strain or age differences in AR or ER expression.
Testosterone has the ability to be aromatized into estrogen by the enzyme aromatase. Thus, testosterone may either work through ARs, ERs, or GPR-30 [44,91,140]. To circumvent the effects of testosterone treatment on estrogen receptors, groups have used 5αDHT, the non-aromatizable androgen. 5αDHT was able to lessen disease severity SJL and C57BL/6 mice, suggesting that at least some of the protective effect of testosterone treatment could be mediated through the AR [26,105]. However, the administration of 5αDHT is not an absolute proof for the involvement of the AR as its metabolite, 3α-androstanediol, which is formed in the brain, may activate ERβ [63,75]. Direct evidence of androgens working through the AR, comes from data showing that testosterone treatment was not able to completely ameliorate disease in the presence of the AR antogonist flutamide in C57BL/6 young male mice [82].
Androgens alter the immune profile of EAE mice. Castration of male SJL mice leads to an increase in immune cell infiltration into the CNS [5]. Evidence has shown that T-cells treated with 5αDHT produced more IL-10 than vehicle treated T-cells [26], suggesting that some anti-inflammatory effects are mediated through the AR. In castrated SJL EAE mice, IFNγ levels were increased in whole spinal cord [5] (Table 1). SJL female T-cells treated with testosterone, from either the spleen or lymph nodes, also showed a decrease in IFNγ with an increase in IL-10 [6]. Further evidence has shown that testosterone treatment decreased TNFα in splenocytes in male mice [82] (Table 1). Perhaps of most importance, SJL mice showed a decrease in adoptive EAE clinical disease when injected with Tcells pre-treated with testosterone versus untreated T-cells [6]. All of this evidence suggests that the androgens may provide some disease protection at least in part through the AR. Further studies are needed to determine what cells might be responsible for this protection.
14. Testosterone and MS
Given that males are less susceptible than females to MS, as well as the animal data demonstrating testosterone’s beneficial effect in EAE, a preliminary study was performed in which men with MS were treated with testosterone. Ten men with RRMS were given a daily treatment of 10 g of a gel containing 100 mg of testosterone for 12 months. This was preceded by a 6-month observation period. Testosterone treatment resulted in a significant slowing of the rate of brain atrophy as measured by monthly MRI. Furthermore, the patients exhibited an improvement in cognitive testing. However, this effect could have been confounded by practice effects in this single arm cross over trial design [128]. PBMCs obtained during the treatment period, as compared to the pre-treatment period, produced significantly more brain-derived neurotrophic factor (BDNF), platelet derived growth factor (PDGF)-BB, and TGFβ-1 with decreased IL-2 production (Table 1). Furthermore, testosterone treatment reduced the DTH recall immune response as well as decreased CD4+ T-cells and increased NK cells [50]. Further study of testosterone treatment in men with MS is warranted.
15. Conclusion
Currently, MS treatments have a primary focus on reducing inflammation. Given that MS is both an inflammatory and neurodegenerative disease, there is a need for neuroprotective treatments if one aims to fully halt the disease [86]. Estrogens and androgens both have the potential to play neuroprotective roles in the treatment of MS. EAE studies with various estrogen and androgen treatments led to clinical disease protection, as well as protection from CNS inflammation, axonal loss, as well as demyelination. ERα ligand treatment was able to completely abrogate both early and late stages of disease. However, it is unlikely ERα ligand treatment could be used long term or in high doses since breast and ovarian cancers are mediated via ERα in humans [12,90]. ERβ ligand and estriol treatments remain promising candidates for MS since they have the potential to be much safer due to either no, or relatively low, binding to ERα respectively [60,63,62,155]. In the EAE model, ERβ ligand was able to ameliorate late signs of clinical disease while providing neuroprotection even in the presence of CNS inflammation [23,141]. Recent evidence suggests that ERβ ligand may not only prevent demyelination, but also promote remyelination [23]. However, since ERβ ligand is does not prevent CNS inflammation, it would theoretically need to be given in combination with an anti-inflammatory agent in MS [32]. Androgen treatment remains a candidate for male MS patients. While some anti-inflammatory effects of testosterone have been shown in EAE, direct neuroprotective effects have not been addressed. It remains unclear whether the protective effect of testosterone might be due to its conversion to estrogen in the CNS.
Despite evidence in the preclinical MS model regarding estrogen’s and androgen’s potential as therapeutic treatments, more research is needed; particularly at the clinical level to assess the potential of sex hormone treatments for MS patients. Two trials are currently under way to test the efficacy estriol and estradiol for women with MS. The first trial is a double blind placebo controlled trial of oral estriol given in combination with a standard of care treatment, Copaxone. RRMS subjects will be treated for 2 years, and the primary outcome measure is a reduction in relapses (http://www.clinicaltrials.gov/ct2/show/NCT00451204). The second trial, POTPART’MUS is a double blind trial aimed to prevent post-partum relapses. Women are given high doses of progestin in combination with physiological doses of estradiol immediately after delivery with continuous administration for the first 3 months post-partum [150]. The field of MS anticipates the results of these trials.
Acknowledgments
Rhonda R. Voskuhl, is supported by the National Institutes of Health (RO1 NS051591, R21 NS071210, and K24NS052117) and the National Multiple Sclerosis Society (CA 1028, RG 4033, and 4364) as well as funding from the Skirball Foundation, the Conrad Hilton Foundation and the Sherak Family Foundation. Rory Spence is supported by an NIH training grant from the UCLA Laboratory of Neuroendocrinology.
References
- 1.Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front. Biosci. 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shammri S, Rawoot P, Azizieh F, AbuQoora A, Hanna M, Saminathan TR, Raghupathy R. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J. Neurol. Sci. 2004;222:21–27. doi: 10.1016/j.jns.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Arevalo M-A, Santos-Galindo M, Bellini M-J, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim. Biophys. Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bebo BF, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 6.Bebo BF, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J. Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 7.Bebo BF, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 8.Bebo BF, Dehghani B, Foster S, Kurniawan A, Lopez FJ, Sherman LS. Treatment with selective estrogen receptor modulators regulates myelin specific T-cells and suppresses experimental autoimmune encephalomyelitis. Glia. 2009;57:777–790. doi: 10.1002/glia.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 10.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur. J. Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogush TA, Dudko EA, Beme AA, Bogush EA, Polotskiĭ BE, Tiuliandin SA, Davydov MI. Estrogen receptor expression in tumors different from breast cancer. Antibiot. Khimioter. 2009;54:41–49. [PubMed] [Google Scholar]
- 13.Brambilla R, Bracchi-Ricard V, Hu W-H, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner T, Evron S, Abramsky O. Effect of experimental autoimmune encephalomyelitis on pregnancy: studies in rabbits and rats. Isr. J. Med. Sci. 1991;27:181–185. [PubMed] [Google Scholar]
- 16.Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camprubí C, Monk D. Does genomic imprinting play a role in autoimmunity? Adv Exp. Med. Biol. 2011;711:103–116. doi: 10.1007/978-1-4419-8216-2_8. [DOI] [PubMed] [Google Scholar]
- 19.Caruso D, D’Intino G, Giatti S, Maschi O, Pesaresi M, Calabrese D, Garcia-Segura L-M, Calza L, Melcangi RC. Sex-dimorphic changes in neuroactive steroid levels after chronic experimental autoimmune encephalomyelitis. J. Neurochem. 2010;114:921–932. doi: 10.1111/j.1471-4159.2010.06825.x. [DOI] [PubMed] [Google Scholar]
- 20.Cho JJ, Iannucci FA, Fraile M, Franco J, Alesius TN, Stefano GB. The role of the estrogen in neuroprotection: implications for neurodegenerative diseases. Neuro Endocrinol. Lett. 2003;24:141–147. [PubMed] [Google Scholar]
- 21.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 23.Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, Voskuhl RR, Tiwari-Woodruff SK. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: historical facts and future implications. Biochim. Biophys. Acta. 2011;1812:177–183. doi: 10.1016/j.bbadis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch. Neurol. 2004;61:886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- 26.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 27.Danel L, Menouni M, Cohen JH, Magaud JP, Lenoir G, Revillard JP, Saez S. Distribution of androgen and estrogen receptors among lymphoid and haemopoietic cell lines. Leuk. Res. 1985;9:1373–1378. doi: 10.1016/0145-2126(85)90125-0. [DOI] [PubMed] [Google Scholar]
- 28.De Paula ML, Rodrigues DH, Teixeira HC, Barsante MM, Souza MA, Ferreira AP. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008;8:1291–1297. doi: 10.1016/j.intimp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp. Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 31.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J. Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 32.Du S, Sandoval F, Trinh P, Voskuhl RR. Additive effects of combination treatment with anti-inflammatory and neuroprotective agents in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du S, Sandoval F, Trinh P, Umeda E, Voskuhl R. Estrogen receptor-β ligand treatment modulates dendritic cells in the target organ during autoimmune demyelinating disease. Eur. J. Immunol. 2011;41:140–150. doi: 10.1002/eji.201040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Etr M, Ghoumari A, Sitruk-Ware R, Schumacher M. Hormonal influences in multiple sclerosis: new therapeutic benefits for steroids. Maturitas. 2011;68:47–51. doi: 10.1016/j.maturitas.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J. Endocrinol. 2005;185:243–252. doi: 10.1677/joe.1.06063. [DOI] [PubMed] [Google Scholar]
- 36.Evron S, Brenner T, Abramsky O. Suppressive effect of pregnancy on the development of experimental allergic encephalomyelitis in rabbits. Am. J. Reprod. Immunol. 1984;5:109–113. doi: 10.1111/j.1600-0897.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 37.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol. Cell. Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 39.García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 41.Gardiner SA, Morrison MF, Mozley PD, Mozley LH, Brensinger C, Bilker W, Newberg A, Battistini M. Pilot study on the effect of estrogen replacement therapy on brain dopamine transporter availability in healthy, postmenopausal women. Am. J. Geriatr. Psychiatry. 2004;12:621–630. doi: 10.1176/appi.ajgp.12.6.621. [DOI] [PubMed] [Google Scholar]
- 42.Garidou L, Laffont S, Douin-Echinard V, Coureau C, Krust A, Chambon P, Guéry J-C. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2004;173:2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- 43.Gatson NN, Williams JL, Powell ND, McClain MA, Hennon TR, Robbins PD, Whitacre CC. Induction of pregnancy during established EAE halts progression of CNS autoimmune injury via pregnancy-specific serum factors. J. Neuroimmunol. 2011;230:105–113. doi: 10.1016/j.jneuroim.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev. Endocr. Metab. Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 45.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell. Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson CL, Gray LJ, Murphy SP, Bath PMW. Estrogens and experimental ischemic stroke: a systematic review. J. Cereb. Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 47.Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc. Natl. Acad. Sci. USA. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog. Brain Res. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 50.Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflamm. 2008;5:32. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold S, Sasidhar M, Morales L, Du S, Sicotte N, Tiwari-Woodruff S, Voskuhl R. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha) Lab. Invest. 2009 doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulshan S, McCruden AB, Stimson WH. Oestrogen receptors in macrophages. Scand. J. Immunol. 1990;31:691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- 53.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, Group MS. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J. Neurol. Neurosurg. Psychiatr. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp. Neurol. 2010;225:9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J. Neuroimmunol. 1994;53:203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 57.Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM. Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol. Endocrinol. 1999;13:440–454. doi: 10.1210/mend.13.3.0255. [DOI] [PubMed] [Google Scholar]
- 58.Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 60.Koehler KF, Helguero LA, Haldosé L-A, Warner M, Gustafsson J-A. Reflections on the discovery and significance of estrogen receptor beta. Endocr. Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 61.Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and puerperium. Ann. Neurol. 1984;16:229–231. doi: 10.1002/ana.410160211. [DOI] [PubMed] [Google Scholar]
- 62.Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and antiestrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 63.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 64.Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J. Neuroimmunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 65.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lélu K, Delpy L, Robert V, Foulon E, Laffont S, Pelletier L, Engelhardt B, Guéry J-C. Endogenous estrogens, through estrogen receptor α, constrain autoimmune inflammation in female mice by limiting CD4(+) T-cell homing into the CNS. Eur. J. Immunol. 2010;40:3489–3498. doi: 10.1002/eji.201040678. [DOI] [PubMed] [Google Scholar]
- 67.Léeu K, Laffont S, Delpy L, Paulet P-E, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry J-C. Estrogen receptor {alpha} signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J. Immunol. 2011 doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 68.Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat. Res. 2005;591:247–263. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 69.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J. Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 70.Littleton-Kearney MT, Ostrowski NL, Cox DA, Rossberg MI, Hurn PD. Selective estrogen receptor modulators: tissue actions and potential for CNS protection. CNS Drug Rev. 2002;8:309–330. doi: 10.1111/j.1527-3458.2002.tb00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- 72.Liu H-B, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen’s immune protection in autoimmune disease. J. Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 73.Lockshin MD. Sex differences in autoimmune disease. Orthop. Clin. North Am. 2006;37:629–633. doi: 10.1016/j.ocl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 75.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J. Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 77.Markovic-Plese S, McFarland HF. Immunopathogenesis of the multiple sclerosis lesion. Curr. Neurol. Neurosci. Rep. 2001;1:257–262. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 78.Martocchia A, Stefanelli M, Cola S, Falaschi P. Sex steroids in autoimmune diseases. Curr. Top. Med. Chem. 2011 doi: 10.2174/156802611796117595. [DOI] [PubMed] [Google Scholar]
- 79.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 80.Matejuk A, Dwyer J, Zamora A, Vandenbark AA, Offner H. Evaluation of the effects of 17beta-estradiol (17beta-e2) on gene expression in experimental autoimmune encephalomyelitis using DNA microarray. Endocrinology. 2002;143:313–319. doi: 10.1210/endo.143.1.8571. [DOI] [PubMed] [Google Scholar]
- 81.Matejuk A, Dwyer J, Hopke C, Vandenbark AA, Offner H. 17Beta-estradiol treatment profoundly down-regulates gene expression in spinal cord tissue in mice protected from experimental autoimmune encephalomyelitis. Arch. Immunol. Ther. Exp. (Warsz) 2003;51:185–193. [PubMed] [Google Scholar]
- 82.Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J. Immunol. 2005;174:2387–2395. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- 83.McClain MA, Gatson NN, Powell ND, Papenfuss TL, Gienapp IE, Song F, Shawler TM, Kithcart A, Whitacre CC. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J. Immunol. 2007;179:8146–8152. doi: 10.4049/jimmunol.179.12.8146. [DOI] [PubMed] [Google Scholar]
- 84.McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- 85.Mcfarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 86.Meuth SG, Bittner S, Ulzheimer JC, Kleinschnitz C, Kieseier BC, Wiendl H. Therapeutic approaches to multiple sclerosis: an update on failed, interrupted, or inconclusive trials of neuroprotective and alternative treatment strategies. BioDrugs. 2010;24:317–330. doi: 10.2165/11537190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Miller SD, Karpus WJ. Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 2007;Unit 15.1, Chapter 15 doi: 10.1002/0471142735.im1501s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 89.Mix E, Meyer-Rienecker H, Hartung H-P, Zettl UK. Animal models of multiple sclerosis - potentials and limitations. Prog. Neurobiol. 2010;92:386–404. doi: 10.1016/j.pneurobio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyoshi Y, Murase K, Saito M, Imamura M, Oh K. Mechanisms of estrogen receptor-α upregulation in breast cancers. Med. Mol. Morphol. 2010;43:193–196. doi: 10.1007/s00795-010-0514-3. [DOI] [PubMed] [Google Scholar]
- 91.Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr. J. 2010;57:101–107. doi: 10.1507/endocrj.k09e-332. [DOI] [PubMed] [Google Scholar]
- 92.Morales LBJ, Loo KK, Liu H-B, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J. Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ngun TC, Ghahramani N, Sánchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front. Neuroendocrinol. 2011;32:227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsen J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008;29:463–475. doi: 10.1016/j.yfrne.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr. Drug Targets CNS Neurol. Disorders. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 96.Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002;58:136–138. doi: 10.1212/wnl.58.1.136. [DOI] [PubMed] [Google Scholar]
- 99.Offner H. Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. J. Neurosci. Res. 2004;78:603–624. doi: 10.1002/jnr.20330. [DOI] [PubMed] [Google Scholar]
- 100.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann. N. Y. Acad. Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 101.Offner H, Vandenbark AA. Congruent effects of estrogen and T-cell receptor peptide therapy on regulatory T cells in EAE and MS. Int. Rev. Immunol. 2005;24:447–477. doi: 10.1080/08830180500371462. [DOI] [PubMed] [Google Scholar]
- 102.Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J. Clin. Invest. 2000;105:1465–1472. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther. 2009;11:252. doi: 10.1186/ar2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 2004;149:84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 105.Palaszynski KM, Loo KK, Ashouri JF, Liu H-B, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 107.Papenfuss TL, Rogers CJ, Gienapp I, Yurrita M, McClain M, Damico N, Valo J, Song F, Whitacre CC. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J. Neuroimmunol. 2004;150:59–69. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 108.Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, Shawler T, Whitacre CC. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J. Immunol. 2011;186:3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res. Dev. Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 110.Pettersson A, Ciumas C, Chirsky V, Link H, Huang Y-M, Xiao B-G. Dendritic cells exposed to estrogen in vitro exhibit therapeutic effects in ongoing experimental allergic encephalomyelitis. J. Neuroimmunol. 2004;156:58–65. doi: 10.1016/j.jneuroim.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, Teuscher C, Vandenbark AA, Offner H. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am. J. Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Polanczyk M, Yellayi S, Zamora A, Subramanian S, Tovey M, Vandenbark AA, Offner H, Zachary JF, Fillmore PD, Blankenhorn EP, Gustafsson J-A, Teuscher C. Estrogen receptor-1 (Esr1) and-2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am. J. Pathol. 2004;164:1915–1924. doi: 10.1016/s0002-9440(10)63752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polanczyk MJ, Jones RE, Subramanian S, Afentoulis M, Rich C, Zakroczymski M, Cooke P, Vandenbark AA, Offner H. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am. J. Pathol. 2004;165:2069–2077. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J. Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 115.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J. Neurosci. Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- 117.Ramirez AD, Liu X, Menniti FS. Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology. 2003;77:223–231. doi: 10.1159/000070277. [DOI] [PubMed] [Google Scholar]
- 118.Ray SK, Samantaray S, Smith JA, Matzelle DD, Das A, Banik NL. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics. 2011;8:180–186. doi: 10.1007/s13311-011-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rieckmann P. Neurodegeneration and clinical relevance for early treatment in multiple sclerosis. Int. MS J./MS Forum. 2005;12:42–51. [PubMed] [Google Scholar]
- 121.Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain. 1995;118(Pt 1):253–261. doi: 10.1093/brain/118.1.253. [DOI] [PubMed] [Google Scholar]
- 122.Saijo K, Collier Jana G, Li Andrew C, Katzenellenbogen John A, Glass Christopher K. An ADIOL-ERβ -CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011:1–22. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samantaray S, Matzelle DD, Ray SK, Banik NL. Physiological low dose of estrogen-protected neurons in experimental spinal cord injury. Ann. N. Y. Acad. Sci. 2010;1199:86–89. doi: 10.1111/j.1749-6632.2009.05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scott E, Zhang Q-g, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front. Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sekigawa I, Fujishiro M, Yamaguchi A, Kawasaki M, Inui A, Nozawa K, Takasaki Y, Takamori K, Ogawa H. A new hypothesis of the possible mechanisms of gender differences in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2010;28:419–423. [PubMed] [Google Scholar]
- 126.Shughrue PJ. Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta) Exp. Neurol. 2004;190:468–477. doi: 10.1016/j.expneurol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 127.Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, Wu TCJ, Voskuhl RR. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 128.Sicotte NL, Giesser BS, Tandon V, Klutch R, Steiner B, Drain AE, Shattuck DW, Hull L, Wang H-J, Elashoff RM, Swerdloff RS, Voskuhl RR. Testosterone treatment in multiple sclerosis: a pilot study. Arch. Neurol. 2007;64:683–688. doi: 10.1001/archneur.64.5.683. [DOI] [PubMed] [Google Scholar]
- 129.Slavin A, Kelly-Modis L, Labadia M, Ryan K, Brown ML. Pathogenic mechanisms and experimental models of multiple sclerosis. Autoimmunity. 2010;43:504–513. doi: 10.3109/08916931003674733. [DOI] [PubMed] [Google Scholar]
- 130.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 2003;171:6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 132.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 133.Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, Teuscher C. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J. Immunol. 2009;182:1789–1793. doi: 10.4049/jimmunol.0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-{alpha} in astrocytes. Proc. Natl. Acad. Sci. USA. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J. Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 136.Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–347. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front. Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Teuscher C, Bunn JY, Fillmore PD, Butterfield RJ, Zachary JF, Blankenhorn EP. Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am. J. Pathol. 2004;165:1593–1602. doi: 10.1016/S0002-9440(10)63416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc. Natl. Acad. Sci. USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tiwari-Woodruff S, Morales LBJ, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc. Natl. Acad. Sci. USA. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trapp BD, Nave K-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 143.Trapp BD, Bö L, Mörk S, Chang A. Pathogenesis of tissue injury in MS lesions. J. Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- 144.Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verdru P, Theys P, D’Hooghe MB, Carton H. Pregnancy and multiple sclerosis: the influence on long term disability. Clin. Neurol. Neurosurg. 1994;96:38–41. doi: 10.1016/0303-8467(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 147.Voskuhl R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. The Neuroscientist : a review journal bringing neurobiology. Neurol. Psyc. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- 149.Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, Sofroniew MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vukusic S, Ionescu I, El-Etr M, Schumacher M, Baulieu EE, Cornu C, Confavreux C P.o.P.-P.R.w.P.a.E.i.M.S.S. Group. The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART’MUS) trial: rationale, objectives and state of advancement. J. Neurol. Sci. 2009;286:114–118. doi: 10.1016/j.jns.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J. Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- 153.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat. Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 154.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Younes M, Honma N. Estrogen receptor β. Arch. Pathol. Lab. Med. 2011;135:63–66. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Q-g, Han D, Wang R-m, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc. Natl. Acad. Sci. USA. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]