Abstract
Afferent feedback alters muscle activity during locomotion and must be tightly controlled. As primary afferent depolarization-induced presynaptic inhibition (PAD-PSI) regulates afferent signaling, we investigated hind limb PAD-PSI during locomotion in an in vitro rat spinal cord-hind limb preparation. We compared the relation of PAD-PSI, measured as dorsal root potentials (DRPs), to observed ipsilateral and contralateral limb endpoint forces. Afferents activated during stance-phase force strongly and proportionately influenced DRP magnitude in the swinging limb. Responses increased with locomotor frequency. Electrical stimulation of contralateral afferents also preferentially evoked DRPs in the opposite limb during swing (flexion). Nerve lesioning in conjunction with kinematic results support a prominent contribution from toe Golgi tendon organ afferents. Thus, force-dependent afferent feedback during stance binds interlimb sensorimotor state to a proportional PAD-PSI in the swinging limb, presumably to optimize interlimb coordination. These results complement known actions of ipsilateral afferents on PAD-PSI during locomotion.
Keywords: presynaptic inhibition, primary afferent depolarization, dorsal root potential, primary afferent, Golgi tendon organ
The circuitry required for generating vertebrate locomotion resides in the spinal cord and is called the locomotor central pattern generator (CPG).1, 2 The locomotor CPG is present at birth in both mouse and rat3. The rodent neonatal spinal cord can be isolated and maintained in vitro4 and, in the presence of bath-applied neuroactive substances, this preparation produces a motor pattern consistent with locomotion as recorded from ventral roots, peripheral nerves, or hind limb muscles.5
Studies in the intact animal indicate that sensory cues related to limb posture and loading play significant roles in determining the spatiotemporal features of motor output.6, 7 Sensory feedback particularly regulates the timing of flexor8, 9 and amplitude of extensor activation.10, 11 Despite the overwhelming importance of sensory feedback to locomotor function, many studies on spinal sensorimotor circuitry are performed in the absence of intact feedback. In order to provide greater similarity to adult locomotion using in vitro preparations, we recently developed a novel preparation – the in vitro neonatal rat spinal cord with hind limbs intact and pendant (SCHIP). This preparation couples natural sensory feedback and behavioral observability with the neural accessibility of the classic in vitro preparations.12 The spinal cord is positioned dorsal-up so that stepping can be activated by bath application of neurochemicals (Fig 1D-E) to generate coordinated muscle activity patterns with limb kinematics that compare well to what is seen in the adult.12-16
Figure 1.
DRPs as measures of primary afferent depolarization and experimental setup. (A) GABA release activates GABAA receptors on intraspinal primary afferent terminals. Afferents possess a high intracellular chloride gradient, leading to chloride efflux. This depolarization travels antidromically as an electrotonic wave where it can be measured as a dorsal root potential (DRP) at the root entry zone. (B) Previous studies have shown that contralateral afferents can evoke PAD-PSI in ipsilateral afferents. FRA = flexor reflex afferents (C) Overhead view of the in vitro SCHIP preparation with exposed spinal cord and intact hind limb. The spinal cord and limbs are maintained in continuously oxygenated artificial cerebrospinal fluid circulated by a peristaltic pump and gravity-fed perfusion system. (D) Recording configuration. DRPs were recorded near the dorsal root entry zones of L2 and/or L5 dorsal roots using glass suction electrodes. Activity in the L2 ventral root (VR) was also recorded. (E) Sagittal view of hind limb-force platform interaction. Two-dimensional force platforms were constructed to monitor forces exerted by a single hind limb.18 (F) Samples recordings of right and left hind limb vertical ground reaction forces measured during locomotion and displayed relative to the right L2 ventral (VR) and right and left dorsal root (DR) activity. Vertical dotted lines mark relative timing of left and right endpoint forces that correspond with DRPs in right and left L2 dorsal roots, respectively. Scale bar is 5 sec. Reproduced in part from Hayes et al.18
We have since incorporated ventral and dorsal root recordings as well as intracellular recordings to relate neural function and behavior in ways not typically possible in vitro.17 A particularly exciting observation regarding afferent feedback concerned the control of transmission in contralateral afferents by a distinctive form of presynaptic inhibition (PSI) called primary afferent depolarization (PAD).18 Such contralateral interlimb control of sensory transmission is barely studied and consequently poorly understood. Yet the magnitude of PAD observed suggests that these control mechanisms are profound.18
Here, we first review the role of afferent feedback on the regulation of ongoing locomotion, with subsequent emphasis on afferent PAD-mediated PSI (PAD-PSI). We then present our previously reported results and provide some additional data on a powerful contralateral afferent evoked PAD-PSI during locomotion. Overall, contralateral stance-phase force feedback may prove to be the most pivotal mechanosensory event encoding sensory gain into the swinging limb during an alternating locomotor gait.
Role of sensory feedback during locomotion
Sensory feedback refines the spatiotemporal features of motor output. Limb extension and loading are primary determinants of phase transition timing.7-9, 19, 20 Overall, it appears that a balance between the excitatory stretch and inhibitory load sensory signals determines the exact timing of the stance-to-swing transition. For example, preventing hip extension hinders swing initiation,8 while stretch or vibration of hip or ankle flexors can alter swing onset timing.9 Hind limb flexor stretch sensitive muscle spindle afferent (Ia afferent) activity during hip extension likely initiates flexion via homonymous and synergistic reflex excitatory feedback. Passive oscillatory hip extensions entrain locomotor speed by altering the duration of stance and the timing of the stance-to-swing transition.21, 22 Loading also controls swing initiation since preventing limb unloading can inhibit flexion generation at the stance-to-swing transition,19, 23 implicating force-sensitive Golgi tendon organ afferent activity (Ib afferents) in loaded extensors.19, 30 The contralateral limb also contributes load-related signals; even when the ipsilateral hip is fully extended, swing will only initiate if the contralateral limb is prepared to accept the load.8, 23
In addition to timing, sensory feedback regulates the magnitude and duration of extensor activity during stance, particularly at the ankle.7, 24-26 In the cat, ankle extensor activity magnitude is reduced if ankle extensor load is reduced while ankle extensor activity and force production increase if ankle extensors are artificially stretched.10, 24, 27 Though responses to length changes are often attributed to Ia muscle spindles, Ib afferents can contribute substantially to ankle extensor activity since Ib feedback onto ankle extensors during locomotion can actually be excitatory to further increase stance-phase extensor activity and force production.10, 26
While less well studied, sensory feedback can also influence swing-phase flexor activity. Resisting hip flexion or stimulation of group I and II afferents in flexor nerves during swing enhances flexor activity,28-30 as does stimulation of toe flexors.31 More generally, stimulation of peripheral nerves during fictive locomotion can reset or entrain centrally-generated rhythms in a task- and phase-dependent manner.29-35
Regulation of sensory inflow by presynaptic inhibition
Precisely because sensory feedback exerts such powerful influence over motor output, it must be tightly regulated. During locomotion, sensory inputs help modify muscle timing and magnitude to meet environmental demands. While both presynaptic and postsynaptic inhibition regulate the effectiveness of sensory transmission onto central circuits,36 their actions are quite different. PSI of intraspinal primary afferent terminals occurs even before the first afferent synapse, so it is well positioned to regulate sensory actions on spinal neurons. Although postsynaptic changes are certainly important and co-exist with PSI effects, PSI is a highly selective and effective way to gate and/or redirect afferent actions.36 PSI can occur via activation of metabotropic or ionotropic receptors. Ionotropic receptor-mediated PSI is a special form of PSI in afferents, typically thought to be caused by to activation of GABAA receptors. Since primary afferents retain high intracellular chloride, GABAA receptor activation leads to a chloride efflux initiating PAD. PAD is thought to reduce transmitter release by inactivating sodium and calcium channels and/or by electrical shunting. In this way, PAD reduces the central actions of incoming sensory events.37, 38 Since PAD travels electrotonically back out the dorsal root toward the periphery, it can be measured experimentally as a dorsal root potential (DRP), and its magnitude monitored as a measure of the relative strength of presynaptic inhibition (Fig 1A).
PAD-PSI encompasses all ensuing descriptions of PSI
Over the past five decades, researchers have characterized the many sources of both afferent and descending PAD-PSI onto group I muscle (Ia and Ib), group II muscle, and cutaneous afferents, both at rest or during fictive locomotion. Most have focused on ipsilateral interactions as provided in a recent review.36 Since we examine actions from contralateral afferents, ipsilateral actions are not described below.
Contralateral contributions to PAD-PSI
Very little attention has been given to contralateral afferent-evoked PAD-PSI. A small number of early studies found that stimulation of group I and flexor reflex afferents produced a contralateral DRP along with the larger ipsilateral DRP.39-41 Figure 1B summarizes these findings. Most contralateral PAD-PSI involves Ib Golgi tendon organ afferents as both the source and receiving afferents.39 In the pentobarbital anesthetized cat, Ib afferents evoked PAD-PSI of contralateral Ib afferents, but Ia afferents neither gave nor received contralateral PAD-PSI, mirroring the largely ipsilateral Ia afferent reflex patterns.39, 42 In the presence of L-DOPA, higher threshold flexor reflex afferents (typically group III not II) could evoke inhibition of contralateral Ia afferents.41 No studies have demonstrated Ia-evoked crossed inhibition under any conditions.
Studies of pedaling on a stationary bike further affirm that sensory inputs from the contralateral limb affect ipsilateral sensory transmission and motor output, particularly of flexors.43, 44 While contralateral movement-related feedback may play a role in ipsilateral sensory regulation, no recent work has investigated crossed PAD-PSI pathways during movements that require interlimb coordination like locomotion.
PAD-PSI during locomotion
While much is known about afferent-evoked PAD-PSI at rest, less is known about PAD-PSI during behavior. It has been difficult to study both centrally-evoked and afferent-evoked PAD-PSI during non-fictive locomotion,45 but PAD-PSI is clearly quite active and phase-dependent during movement.46, 47 DRPs and intra-axonal PAD are rhythmic during fictive locomotion, confirming actions independent of rhythmic afferent feedback.e.g. 48, 49 Locomotor-related PAD-PSI is typically maximal during the flexion phase in the majority of group I and II muscle as well as cutaneous afferents.49, 50 While afferent-evoked PAD-PSI appears to be more effective than locomotor-related PAD-PSI,51 these circuitries interact since locomotor circuits modulate the effectiveness of sensory-evoked PAD-PSI in a phase-dependent and muscle-dependent manner.45, 52
Thus, the spinal locomotor circuitry and recruited afferents interact to create dynamic patterns of presynaptic sensory regulation. An important goal therefore is to understand these interactions during behaviorally-relevant natural patterns of afferent activity. The SCHIP allowed us to mechanically isolate the spinal cord from the limbs to provide sufficient stability for study of contralateral and ipsilateral PSI-PAD during non-fictive locomotion while manipulating the mechanics and neural system in ways otherwise not possible.
Methods
The development of SCHIP and the results obtained have been described previously.17, 18, 53 Briefly, locomotion was induced with N-methyl-d-aspartate and serotonin with or without dopamine added to the artificial cerebrospinal fluid solution. Activity in the lumbar L2 or L5 ventral roots was recorded as a monitor of spinal motor output. Bursting activity in these roots corresponds to flexor and extensor locomotor phases, respectively.14 DRPs were recorded with glass suction electrodes placed en passant near the entry zones of dorsal roots L2 and/or L5. DRPs are a measure of PAD-PSI and DRP amplitude increases indicate increases in PAD-PSI. Limb endpoint forces were measured using two 2D force platforms, one for each hind limb. A description of the preparation and obtained recordings has been presented previously18 and is summarized in Figure 1C-F. Throughout the description in Results, unless otherwise stated ipsilateral indicates the side of the recorded DRP (ipsi-DRP) and contralateral indicates the side contralateral to the DRP.
Results
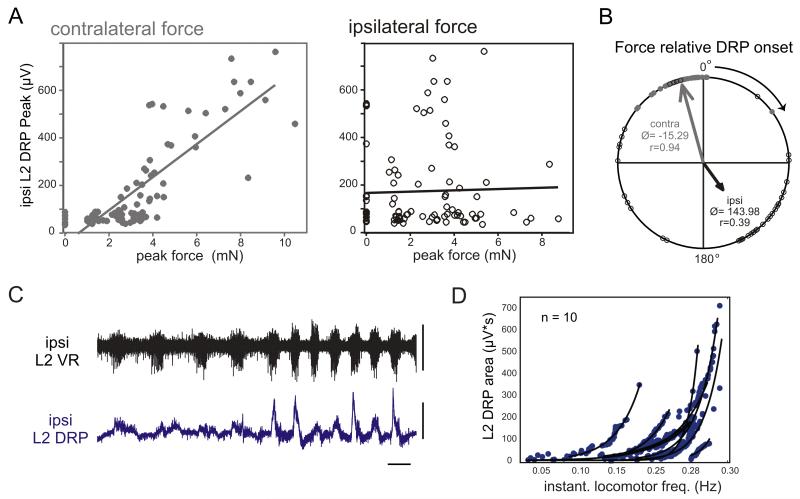
Figure 1F shows the typical locomotor patterns of L2 DRPs recorded from dorsal roots (DRs) in relationship to L2 ventral root (VR) and ipsilateral and contralateral forces. Note that contralateral foot contact forces predicted timing of the ipsilateral DRPs (left force on right DRP, right force on left DRP). DRP amplitude also appeared to scale with contralateral force. Indeed, when L2 DRP area, mean amplitude or peak amplitude are plotted against the corresponding values for the ipsilateral and contralateral forces in each cycle, significant correlations are only observed with contralateral force (n = 10/10 for area, 9/10 for peak and 8/10 for mean). None showed a significant positive correlation with ipsilateral force magnitude (Fig. 2A).18
Figure 2.
Ipsilateral L2 DRP strength scales with contralateral force and locomotor frequency. (A) Linear regression relating DRP area to contralateral (left panel; grey dots) and ipsilateral peak forced (middle panel; open circles) from a representative animal. Each point represents a single cycle (n = 95 cycles). DRP peak was strongly correlated with contralateral force (R = 0.83, P < 0.0001) but not ipsilateral force (R = 0.03; P = 0.79). Note that when no contralateral force occurs (grey points on the y-axis), the DRP is small or zero, but the absence of ipsilateral force (left; open circle points on the y-axis) does not affect DRP magnitude. (B) Phase relationship of contralateral and ipsilateral force relative to DRP onset. 0° represents the DRP onset and the cycle progresses clockwise from 0° (in-phase) to 180° (out-of-phase) to 360°/0°. Northwest points precede DRP onset, northeast points lag. Arrow length represents the concentration (r) about the mean angle (Ø). Note contralateral force consistently precedes DRP onset while ipsilateral force is typically out of phase with the DRP and contralateral force. (C) In this animal, a perturbation induced a rapid increase in frequency and force in the middle of a locomotor bout. As a result, L2 DRP amplitude rapidly increased, highlighting the increase in PAD-PSI with locomotor frequency and force. Scale bars: 5 sec, 200 μV. (D) DRP area versus instantaneous locomotor frequency fitted by exponential curves for 10 animals. Note that DRP area increases with increasing locomotor frequency. Reproduced from Hayes et al.18
Within a step cycle, the contralaterally-evoked ipsilateral DRP occurred during the early ipsilateral flexion phase in all animals. In order for the contralateral limb to evoke or influence DRP magnitude, and thus the amount of PAD-PSI on the ipsilateral limb, contralateral force must precede the onset of the ipsilateral L2 DRP during each cycle. Figure 2B shows the phasing of the ipsilateral and contralateral force onset relative to DRP onset for a typical bout of locomotion. Note that contralateral force immediately preceded the ipsilateral DRP while ipsilateral force timing was more variable and out-of-phase. The mean onset of contralateral force always just preceded the onset of the DRP (n = 10/10). Overall, we observed that the timing of PAD-PSI is tightly coupled to contralateral limb endpoint force, but neither ipsilateral force nor motor output timing. When locomotor frequency increased, the magnitude of the DRP also increased (Fig. 2C). The relationship between L2 DRP area and locomotor frequency was generally well fit with an exponential curve suggesting that sensory feedback is progressively depressed as locomotor frequency increased (n = 10/10 with R2 > 0.85; Fig 2D). In comparison, there was no relationship between DRP magnitude and any hind limb kinematic variable compared (contralateral ankle, knee, and hip range of motion, area under the angular trajectory, magnitude of concurrent ipsilateral flexion and extension).18
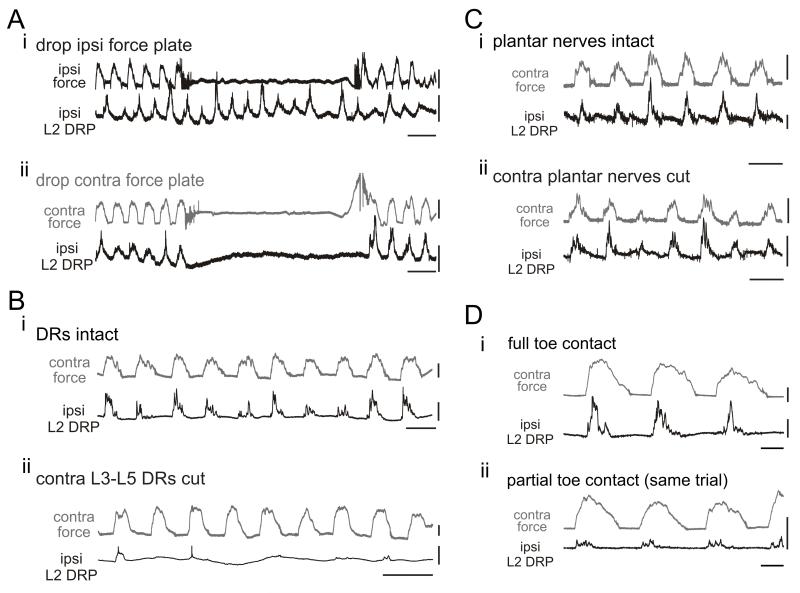
Several perturbations further confirmed the source of the ipsilateral DRP (Fig 3.). To establish the essential requirement for contralateral endpoint force in ipsilateral DRP generation, ipsilateral and contralateral plates were separately removed (Fig. 3A). While ipsilateral DRPs were largely unchanged when the ipsilateral plate was removed (n = 6/7), DRPs were clearly reduced or abolished during contralateral plate removal (n = 7/8). These changes were not associated with changes in locomotor frequency. To confirm that contralateral afferents were responsible for evoking the force-related DRPs, contralateral lumbar L3-L5 dorsal roots were cut and DRPs were abolished or reduced in number and consistency in 3/4 experiments (Fig. 3B). Surprisingly, cutting the contralateral plantar nerve to remove most plantar surface cutaneous afferents did not affect ipsilateral DRP generation (n = 3/3; Fig. 3C) suggesting that cutaneous afferents are not primarily involved in the generation of force-dependent DRPs. To explore this further we observed that during locomotion, if the foot contacted the plate proximal to the metatarsophalangeal joint (no toe contact) DRPs were reduced or absent (n = 3; Fig. 3D). This strongly implicates toe afferents as primarily responsible for the crossed DRP.18
Figure 3.
Response to contralateral plate removal and dorsal root rhizotomy and plantar nerve transection.(A) Ipsilateral DRPs are shown with ipsilateral force (Ai) and contralateral force (Aii). Note lack of locomotor frequency change before, during, or after plate removal. (Ai) When the ipsilateral plate was removed, the ipsilateral DRP was largely unchanged. (Aii) When the contralateral plate was removed, reducing contralateral limb loading, the ipsilateral L2 DRP was nearly abolished. The DRPs returned as soon as the contralateral plate was restored. Scale bars are 10 mN, 200 μV, and 10 sec. (B) L2 DRP relative to contralateral force during locomotion with dorsal roots intact. (Bi) Ipsilateral L2 DRP relative to contralateral force during locomotion with dorsal roots intact. (Bii) Following rhizotomy of contralateral L3-5 dorsal roots, the DRP was significantly reduced and inconsistent despite high contralateral forces. The frequency increase seen is due to the addition of 2 μM NMDA to induce locomotion without intact roots. Scale bars are 10 mN, 400 μV, and 5 sec. (C) Ipsilateral L2 DRP and contralateral force before and after plantar nerve transection. (Cii) DRP persists following transection of the contralateral medial and lateral plantar nerves and continues to scale with contralateral force. Scale bars are 20 mN, 800 μV, and 5 sec. (D) Contralateral toe contact and observed DRPs. (Di) L2 DRP with full contralateral toe contact (Dii) Later in the same locomotor bout, the toe moved forward so only a small portion contacted the plate. This resulted in reduced amplitude DRPs. Scale bars are 400 μV, 5 mN, and 2 s. Reproduced from Hayes et al.18
Previously, ipsilateral afferent stimulation-evoked PAD was shown to be maximal during the flexion phase of fictive locomotion in the majority of low threshold afferents.49, 50 Here, we undertook the first experiments that test whether a similar phase dependent organization applies to contralateral afferents. To demonstrate that the amplitude of afferent stimulation-evoked crossed DRPs is locomotor phase dependent, we stimulated contralateral dorsal roots at five times the intensity of afferent volley detection threshold (5T) at 30-60 sec intervals. We examined evoked responses throughout the locomotor cycle either with the force plate positioned to optimize limb contact at stance (Fig. 4A) or for minimized footcontact (Figure 4B). In both cases, electrical stimulation of right limb afferents during the onset of right limb extension generated a strong DRP on the opposite side, presumably due to activation of afferents normally recruited via limb loading. In comparison, afferent stimulation at the end of extensor phase or during the flexor phase did not reliably evoke crossed DRPs (n = 3/3). Overall, the crossed PAD generated by ground contact would coincide with ipsilateral PAD, previously reported to be maximal during ipsilateral flexion49, 50. Complementary bilateral actions could serve to reinforce afferent-evoked PAD-PDI, perhaps via actions on common interneurons.
Figure 4.
The amplitude of afferent stimulation-evoked crossed DRPs is locomotor phase dependent. Shown are results from two animals (A & B). Right (contralateral) lumbar dorsal roots were stimulated at 5T. Simultaneous individual left dorsal root (DR), left ventral root (VR), and right force recordings are matched in vertical progression in all panels as indicated with numbers on left records in (A) and (B). Arrows at top identify stimulus onset for each panel. (Ai) Electrical stimulation of right DRs near onset of right extension produced large crossed DRPs that corresponded with force plate interactions (extensor phase timing is inferred from left flexor-related L2VR motor activity). (ii) During the end of the extensor phase (ext. end), electrical stimulation could still produce comparable plate contact force increases as in (i), but did not recruit DRPs (upper two raw records in blue), while stimulation during right limb flexion (flex) evoked only small DRPs and was not associated with any force increases (bottom two raw records). (B) In this experiment, the force plate was positioned to minimize foot contact during right limb extension. Evoked responses were then separated into 3 epochs (i-iii) to compare the relative crossed DRP amplitudes during various phases of locomotion (locomotor phase timing is inferred from right extensor-related L5VR motor activity). Green vertical highlighting identifies a one second period post-stimulation. Vertical boxed regions denote subsequent bouts of right extensor activity. (i) Stimulation of right afferents during onset of right limb extension evoked a strong DRP in the opposite limb, presumably due to activation of afferents normally recruited via limb loading. In comparison, afferent stimulation at the end of extensor (ii) or during the flexor phase (iii) did not reliably evoke crossed DRPs. (iii) Flexor phase afferent stimulation appeared to enhance the subsequent extensor phase (boxed region) such that limb extension now generated larger foot contact forces with correspondingly large evoked crossed DRPs. Scale bars are 400 μV (for DR), 1 sec.
Discussion
Summary
We recorded DRP activity as a measure of PAD-PSI and compared the spatiotemporal dependence of DRP patterns on ipsilateral and contralateral limb force and kinematics. We performed mechanical perturbations on each limb to isolate the influence of the individual limbs and distinguish between movement- and force-related feedback. Because both central circuits and sensory feedback can influence PAD-PSI, we also considered the dependence of PAD-PSI on locomotor phase, motor output, and performed deafferentations to distinguish central and sensory sources. We found that the mechanics of the contralateral limb, particularly limb loading, played a pivotal role in regulating ipsilateral sensory inflow via contralateral afferent-evoked PAD-PSI. One possible purpose for contralaterally-generated force-sensitive presynaptic inhibition is to preserve swing by inhibiting unwanted afferent inputs that counteract flexion.
Figure 5 provides a proposed circuitry to explain the underlying PAD-PSI, and the numbers below correspond to sites of action in this figure. We hypothesize that contralateral limb loading activates Ib Golgi tendon organs in extrinsic toe flexors (1) whose afferents project via lumbar dorsal roots to activate first-order glutamatergic excitatory commissural interneurons (2)63 that subsequently activate last-order GABAergic interneurons (3) responsible for generating the observed PAD-PSI. The net effect is to inhibit sensory input from the ipsilateral swinging limb during flexor motor output (4). The timing is consistent with observations of ipsilateral afferents also producing peak ipsilateral PAD-PSI during limb flexion.49, 50 The crossed PAD-PSI pathway is likely under control from the locomotor CPG (5) since the magnitude of its interlimb actions are suppressed when the limb is not in stance (Fig. 4).
Figure 5.
Proposed circuitry underlying the contralateral presynaptic inhibition (PAD-PSI) evoked by contralateral limb loading. Sensory events and dorsal roots are shown in gray. Motor output is shown in black. A simplified representation of the central pattern generator (CPG) is also shown. Identities of events are numbered 1-5. (1) Contralateral limb loading and toe contact activate Ib afferents that then enter contralateral lumbar dorsal roots. The magnitude of afferent activation scales with force. (2) Since the majority of identified commissural interneurons receiving Ib input are glutamatergic,63 these afferents likely activate glutamatergic commissural interneurons (Glu). (3) Glu interneurons subsequently activate GABAergic interneurons (GABA), producing crossed PAD-PSI; recorded as a DRP at ipsilateral lumbar dorsal roots. (4) By blocking sensory inputs and/or closing sensory pathways, PAD-PSI may impact ipsilateral motor output during the swing/flexion phase. (5) Expression of crossed PAD-PSI is locomotor phase dependent and depressed during the flexion phase.
Identity of afferent populations recruited during ground contact and undergoing PAD-PSI
We observed that the crossed flexion-phase DRPs scaled with contralateral afferent signaling. The identity of the afferent modalities responsible is herein considered. First, cutaneous mechanoceptors on the paw plantar surface are not likely responsible since removing most of its innervation by cutting the plantar nerve did not reduce the DRPs. However a cutaneous afferent contribution cannot be excluded since a small cutaneous receptive field on digits two and three remains after plantar nerve section.54, 55 Plantar nerve section also largely denervates intrinsic toe muscles, arguing against their contribution to contralateral PAD-PSI.
While the overall loading of muscles during ground contact undoubtedly contributes to PAD-PSI, toe contact clearly contributed strongly to the evoked DRPs. Extrinsic toe extensors, such as extensor digitorum longus and extensor hallucis longus (toe extensors/ankle flexors) are unlikely involved as they are most active during swing, and their Ia and Ib afferents tend to fire in early swing rather than stance.56, 57 In contrast, extrinsic toe flexors, particularly flexor hallucis longus (FHL, toe flexors/ankle extensors), are active during stance with other ankle extensors.58 Flexor digitorum longus (FDL) can be coactivated with FHL and other ankle extensors in the rat,14 and recordings from afferents in the moving cat show that FHL and FDL group Ia and Ib afferents are both active during stance, particularly at toe contact.56, 57, 59 This makes their peak firing well-timed to evoke the observed patterns of PAD-PSI. As some joint afferents signal midrange joint angles, and toe joint afferents are involved in proprioception, joint afferents may also contribute to the PAD-PSI seen.60
Overall, based on the known contralateral PAD-PSI pathways reviewed earlier, Ib afferents from FHL and FDL seem the most likely afferent source of contralateral force-sensitive PAD-PSI.39 Indeed, increased contralateral limb endpoint force would likely increase Ib firing as ankle extensor/toe flexor activity increases,10, 56 readily explaining the scaling of PAD-PSI with contralateral force. Because most quadrupeds walk digitigrade,61 the toe muscles are also well positioned to sense ground stability and unexpected toe and ankle perturbations during stance, making their contribution to contralateral sensory regulation positionally appropriate, along with a contribution from toe muscles.
The afferents possibly receiving contralateral PAD-PSI include Ia, Ib, cutaneous and joint afferents. Ib afferents are again the most likely since they are known to receive crossed PAD-PSI from contralateral Ib afferents.39 In contrast, cutaneous and Ia afferents have not been shown to receive crossed PAD-PSI from low-threshold muscle afferents.39, 62
Overall, this work has identified a stance force-encoded crossed PAD-PSI modulated by locomotor phase. While a prominent contribution from Ib afferents is suggested, additional studies are required to more precisely identify recruited afferents. In comparison, nothing is currently known about the identity of the ipsilateral afferents receiving the crossed input to generate PAD-PSI. Identification of these afferents, interposed interneuronal pathways, and interactions both with ipsilaterally-evoked PAD-PSI and the locomotor CPG are needed to more fully understand the interaction between sensory and locomotor systems.
Acknowledgments
This work was funded by NIH NS45248, NIH NS65949, and NSF 0745164 to S.H., NIH AR054760 to Y.H.C., and NSF GRFP fellowship to H.B.H.
References
- 1.Brown TG. The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society of London. 1911;84:308–319. [Google Scholar]
- 2.Grillner S. Handbook of Physiology-The Nervous System II. 1981. Control of locomotion in bipeds,tetrapods, and fish; pp. 1179–1236. [Google Scholar]
- 3.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu.Rev.Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 4.Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain research bulletin. 2000;53:661–669. doi: 10.1016/s0361-9230(00)00399-3. [DOI] [PubMed] [Google Scholar]
- 5.Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 1988;2:2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- 6.Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos.Trans.R.Soc.Lond B Biol.Sci. 2006;361:1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Annals of the New York Academy of Sciences. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- 8.Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain research. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- 9.Hiebert GW, et al. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of neurophysiology. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- 10.Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. Journal of neurophysiology. 2004;92:2093–2104. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. Journal of Neurophysiology. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- 12.Hayes HB, Chang YH, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. Journal of neurophysiology. 2009;101:1114–1122. doi: 10.1152/jn.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juvin L, Simmers J, Morin D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J.Neurosci. 2005;25:6025–6035. doi: 10.1523/JNEUROSCI.0696-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J.Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- 15.Klein DA, Tresch MC. Specificity of intramuscular activation during rhythms produced by spinal patterning systems in the in vitro neonatal rat with hindlimb attached preparation. Journal of neurophysiology. 2010;104:2158–2168. doi: 10.1152/jn.00477.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein DA, Patino A, Tresch MC. Flexibility of motor pattern generation across stimulation conditions by the neonatal rat spinal cord. Journal of neurophysiology. 2010;103:1580–1590. doi: 10.1152/jn.00961.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochman S, et al. Enabling techniques for in vitro studies on mammalian spinal locomotor mechanisms. Frontiers in bioscience: a journal and virtual library. 2012;17:2158–2180. doi: 10.2741/4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes HB, Chang YH, Hochman S. Stance-phase force on the opposite limb dictates swing-phase afferent presynaptic inhibition during locomotion. J Neurophysiol. 2012;107:3168–3180. doi: 10.1152/jn.01134.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- 20.Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Experimental Brain Research. 1995;103:20–30. doi: 10.1007/BF00241961. [DOI] [PubMed] [Google Scholar]
- 21.Andersson O, Grillner S. Peripheral control of the cat’s step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during “fictive locomotion.”. Acta Physiol Scand. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- 22.Kriellaars DJ, et al. Mechanical entrainment of fictive locomotion in the decerebrate cat. J.Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- 23.Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. The Journal of physiology. 2000;528(Pt 2):389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate Cat. Journal of neurophysiology. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- 25.Juvin L, Simmers J, Morin D. Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators. J.Physiol. 2007;583:115–128. doi: 10.1113/jphysiol.2007.133413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- 27.Gorassini MA, et al. Corrective responses to loss of ground support during walking. I. Intact cats. Journal of neurophysiology. 1994;71:603–610. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- 28.Lam T, Pearson KG. Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol. 2001;86:1321–1332. doi: 10.1152/jn.2001.86.3.1321. [DOI] [PubMed] [Google Scholar]
- 29.Perreault MC, et al. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. Journal of Physiology. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quevedo J, et al. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol. 2000;525(Pt 2):549–564. doi: 10.1111/j.1469-7793.2000.t01-1-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stecina K, Quevedo J, McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. The Journal of physiology. 2005;569:275–290. doi: 10.1113/jphysiol.2005.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- 33.Iizuka M, Kiehn O, Kudo N. Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Experimental Brain Research. 1997;114:193–204. doi: 10.1007/pl00005628. [DOI] [PubMed] [Google Scholar]
- 34.Kiehn O, Iizuka M, Kudo N. Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci.Lett. 1992;148:43–46. doi: 10.1016/0304-3940(92)90800-m. [DOI] [PubMed] [Google Scholar]
- 35.Quevedo J, et al. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol. 2005;94:2045–2052. doi: 10.1152/jn.00175.2005. [DOI] [PubMed] [Google Scholar]
- 36.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- 37.Eccles JC, Kostyuk PG, Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol (Paris) 1962;161:237–257. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eccles JC. PRESYNAPTIC INHIBITION IN THE SPINAL CORD. Prog.Brain Res. 1964;12:65–91. doi: 10.1016/s0079-6123(08)60618-4. [DOI] [PubMed] [Google Scholar]
- 39.Devanandan MS, Holmqvist B, Yokota T. Presynaptic Depolarization of Group I Muscle Afferents by Contralateral Afferent Volleys. Acta Physiol Scand. 1965;63:46–54. doi: 10.1111/j.1748-1716.1965.tb04040.x. [DOI] [PubMed] [Google Scholar]
- 40.Gossard JP, Rossignol S. Phase-dependent modulation of dorsal root potentials evoked by peripheral nerve stimulation during fictive locomotion in the cat. Brain Research. 1990;537:1–13. doi: 10.1016/0006-8993(90)90333-7. [DOI] [PubMed] [Google Scholar]
- 41.Jankowska E, Lund S, Lundberg A. The effect of DOPA on the spinal cord 4. Depolarization evoked in the contralateral terminals of contralateral Ia afferent terminals by volleys in the flexor reflex afferents. Acta Physiologica Scandinavica. 1966;68:337–341. [Google Scholar]
- 42.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 43.Alibiglou L, et al. Bilateral limb phase relationship and its potential to alter muscle activity phasing during locomotion. J Neurophysiol. 2009;102:2856–2865. doi: 10.1152/jn.00211.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ting LH, et al. Bilateral integration of sensorimotor signals during pedaling. Annals of the New York Academy of Sciences. 1998;860:513–516. doi: 10.1111/j.1749-6632.1998.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 45.Menard A, Leblond H, Gossard JP. The modulation of presynaptic inhibition in single muscle primary afferents during fictive locomotion in the cat. J Neurosci. 1999;19:391–400. doi: 10.1523/JNEUROSCI.19-01-00391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yakhnitsa PA, IA, Bulgakova NV. Phase-dependent changes in dorsal root potential during actual locomotion in rats. Neirofiziologiya. 1988;20:333–340. [PubMed] [Google Scholar]
- 47.Beloozerova IN, Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. II: studies during treadmill locomotion. Brain Res. 2004;996:227–236. doi: 10.1016/j.brainres.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 48.Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res. 1988;70:15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- 49.Gossard JP, Cabelguen JM, Rossignol S. An intracellular study of muscle primary afferents during fictive locomotion in the cat. J.Neurophysiol. 1991;65:914–926. doi: 10.1152/jn.1991.65.4.914. [DOI] [PubMed] [Google Scholar]
- 50.Gossard JP, Cabelguen JM, Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol. 1989;62:1177–1188. doi: 10.1152/jn.1989.62.5.1177. [DOI] [PubMed] [Google Scholar]
- 51.Gossard JP. Control of transmission in muscle group IA afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1996;76:4104–4112. doi: 10.1152/jn.1996.76.6.4104. [DOI] [PubMed] [Google Scholar]
- 52.Menard A, Leblond H, Gossard JP. Modulation of monosynaptic transmission by presynaptic inhibition during fictive locomotion in the cat. Brain Res. 2003;964:67–82. doi: 10.1016/s0006-8993(02)04067-2. [DOI] [PubMed] [Google Scholar]
- 53.Hayes HB, Chang YH, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. J.Neurophysiol. 2009;101:1114–1122. doi: 10.1152/jn.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol. 2003;90:3625–3639. doi: 10.1152/jn.00496.2003. [DOI] [PubMed] [Google Scholar]
- 55.Holmberg H, Schouenborg J. Developmental adaptation of withdrawal reflexes to early alteration of peripheral innervation in the rat. J Physiol. 1996;495(Pt 2):399–409. doi: 10.1113/jphysiol.1996.sp021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loeb GE, Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979;42:420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- 57.Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998;507(Pt 1):293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Donovan MJ, et al. Actions of FDL and FHL muscles in intact cats: functional dissociation between anatomical synergists. J Neurophysiol. 1982;47:1126–1143. doi: 10.1152/jn.1982.47.6.1126. [DOI] [PubMed] [Google Scholar]
- 59.Prochazka A, Westerman RA, Ziccone SP. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976;39:1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- 60.Ferrell WR. The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of movement. J Physiol. 1980;299:85–99. doi: 10.1113/jphysiol.1980.sp013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham CB, et al. The influence of foot posture on the cost of transport in humans. J Exp Biol. 2010;213:790–797. doi: 10.1242/jeb.038984. [DOI] [PubMed] [Google Scholar]
- 62.Baldissera F, Hultborn H, Illert M. Handbook of Physiology. Section I: The Nervous System. Williams and Wilkins; Baltimore: 1981. Integration in spinal neuronal systems; pp. 509–595. [Google Scholar]
- 63.Bannatyne BA, et al. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J.Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]