Figure 3.
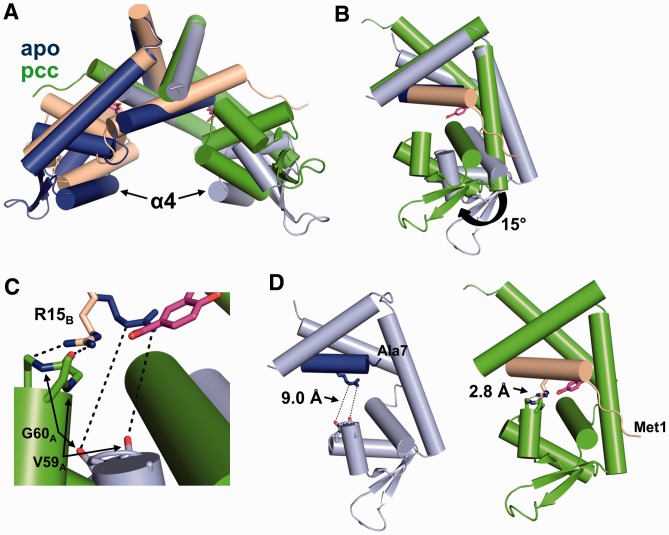
Ligand binding induces significant conformational changes in PcaV. (A) Secondary structure superposition of the apo-Pcav dimer (colored in light blue and dark blue) and the PcaV–PCA dimer (colored as in Figure 2). (B) Superposition of the dimerization core of one monomer of apo-PcaV and ligand-bound PcaV (colored as in A). The PcaV wHTH domain rotates up 15° towards helix α1 of the opposite monomer on ligand binding to form the PCA-binding pocket. (C) Zoomed-in view of the interactions between Arg15 (shown as stick representation in blue and beige for apo- and ligand-bound PcaV, respectively) and the backbone carbonyl groups from Val59 and Gly60 from the opposite monomer (apo-PcaV in light blue and ligand-bound PcaV in green). (D) Orientation of Arg15B relative to Val59A and Gly60A in the apo (left panel) and PCA-bound (right panel) structures. In apo-PcaV, Arg15B is 9 Å away from the loop preceding helix α4 of the opposite monomer. Rotation of the wHTH domain on ligand-binding brings Arg15B in close proximity to the Val59A-Gly60A loop, forming polar interactions that stabilize the ligand-bound conformation.