Scheme 4.
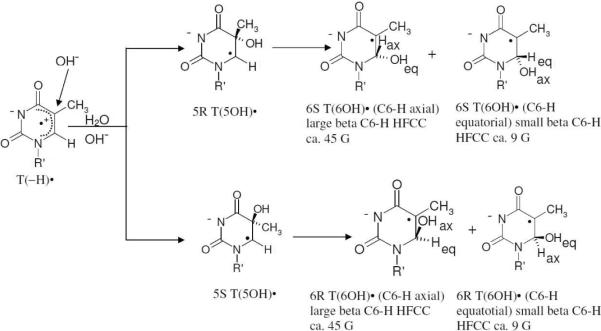
Addition of OH− to C5 of T(−H)• yields T(5OH)• (5R and/or 5S). Subsequently, T(5OH)• is converted to various diastereomers of T(6OH)•. The R and S steroisomers of T(6OH)• can not be distinguished by ESR spectroscopy and only the axial and equatorial conformers can be distinguished on the basis of β C6-H HFCC values whereas for T(5OH)• only C6αH hyperfine coupling is experimentally observable and it is same (ca. 20 G) for R or S.
