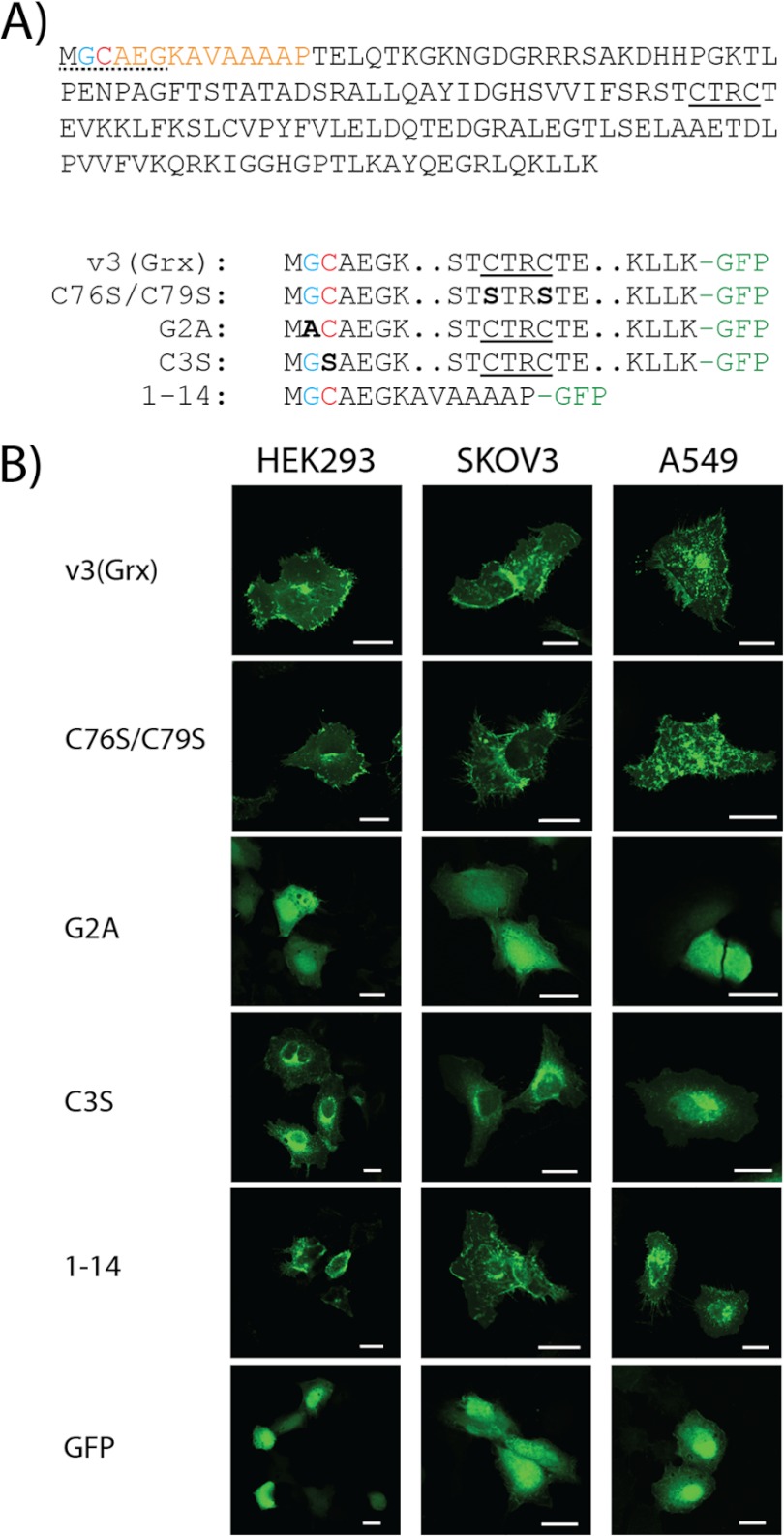
FIGURE 1.
The redox active site dithiol motif of v3 is dispensable, whereas its N-terminal acylation motif is required for membrane association of the v3(Grx) domain. A, the Grx domain of v3 is here shown with the proposed consensus sequence for myristoylation underlined (dotted; presumed myristoylated Gly residue shown in blue) at the beginning of a 14-amino acid stretch at the N terminus of the protein (orange). A Cys-3 residue may potentially be palmitoylated (red). The dithiol active site motif of v3(Grx) is underlined (solid). Final amino acid sequences of the v3(Grx)-derived constructs studied herein are schematically indicated below. Mutated residues are marked bold and in black with the names of the variants to the left. Two dots indicate omitted residues (for full sequence, see top panel), and -GFP in green indicates a C-terminal GFP fusion partner. B, A549, HEK293, and SKOV3 cells were transfected using either of the v3(Grx)-derived GFP fusion constructs: wild type v3(Grx), the active site mutant C76S/C79S, the N-terminal G2A or C3S mutants, or a truncated variant solely containing the first 14 amino acids of v3 (1–14). Control transfections were performed using a construct expressing GFP alone (GFP). Fluorescence was recorded 18 h after transfection using confocal microscopy. Scale bar = 20 μm.