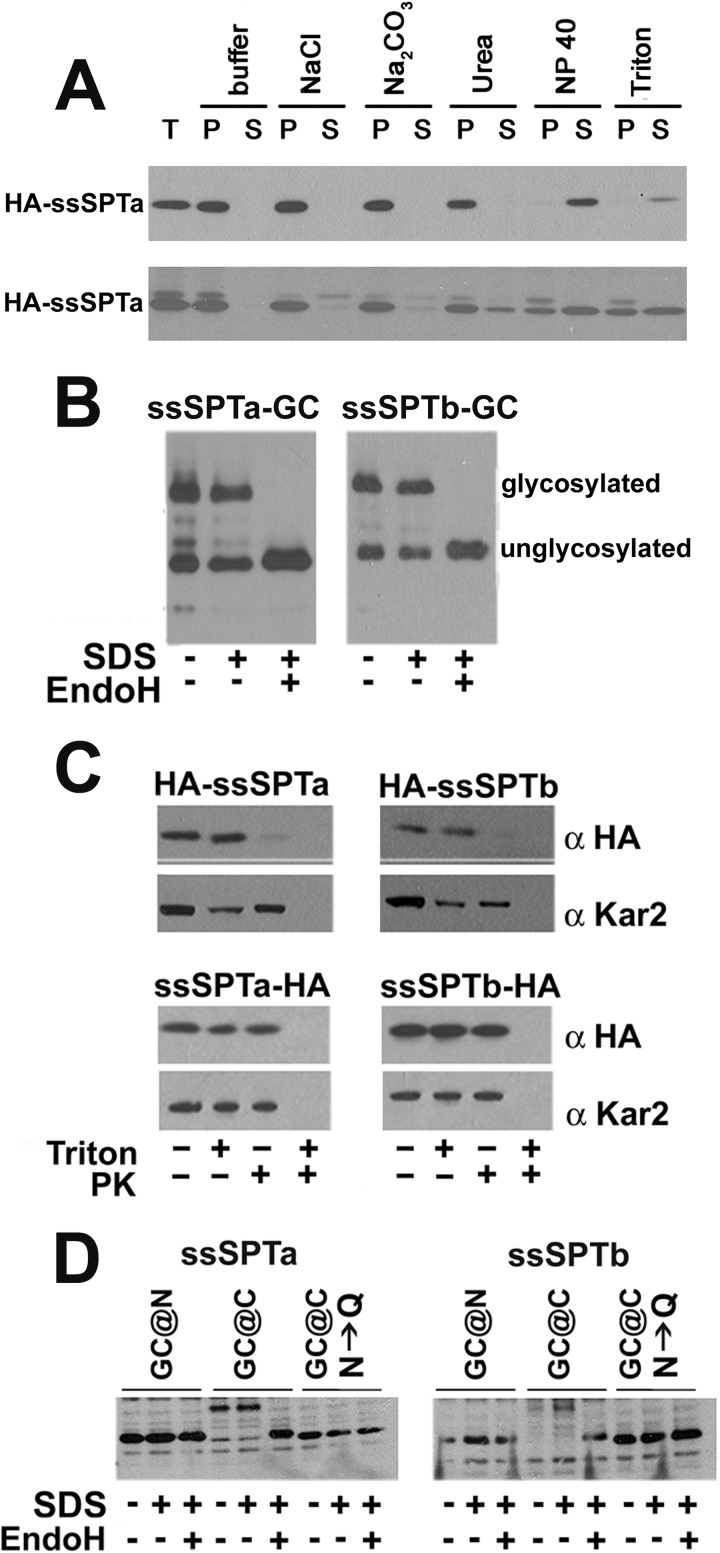
FIGURE 2.
The hssSPTs are integral ER membrane proteins with their N termini in the cytosol and C termini in the lumen. A, microsomes prepared from yeast (upper) or CHO-Ly-B cells (lower) expressing HA-hssSPTa, hLCB1 and hLCB2a were extracted on ice with an equal volume of TEGM or TEGM containing 1 m NaCl, 5 m urea, 0.2 m Na2CO3, 0.4% Nonidet P-40 or 2% Triton X-100 for 60 min. The samples were subjected to centrifugation at 100,000 × g for 30 min, and equal proportions of the supernatants and pellets were resolved by SDS-PAGE. HA-ssSPTa was detected by immunoblotting with anti-HA antibody. B, ssSPTa and ssSPTb containing an N-terminal HA tag and a C-terminal GC cassette were expressed in yeast along with hLCB1 and hLCB2a. Microsomal protein (12.5 μg with or without EndoH treatment) was resolved by SDS-PAGE, and ssSPTa was detected by immunoblotting. C, sealed microsomal vesicles were prepared from yeast expressing N- or C-terminally HA-tagged ssSPTa or ssSPTb, hLCB1, and hLCB2a. The microsomes were mock digested or digested with proteinase K (PK) in the absence or presence of Triton X-100. The luminal ER protein, Kar2p, served as a control for vesicle integrity. D, the N- or C-terminally GC-tagged ssSPTs were expressed along with hLCB1 and hLCB2a in CHO-Ly-B cells and assessed for glycosylation as described above. GC cassettes in which the asparagines in the consensus glycosylation sites were mutated to glutamines (N→Q) were used as controls.