FIGURE 3.
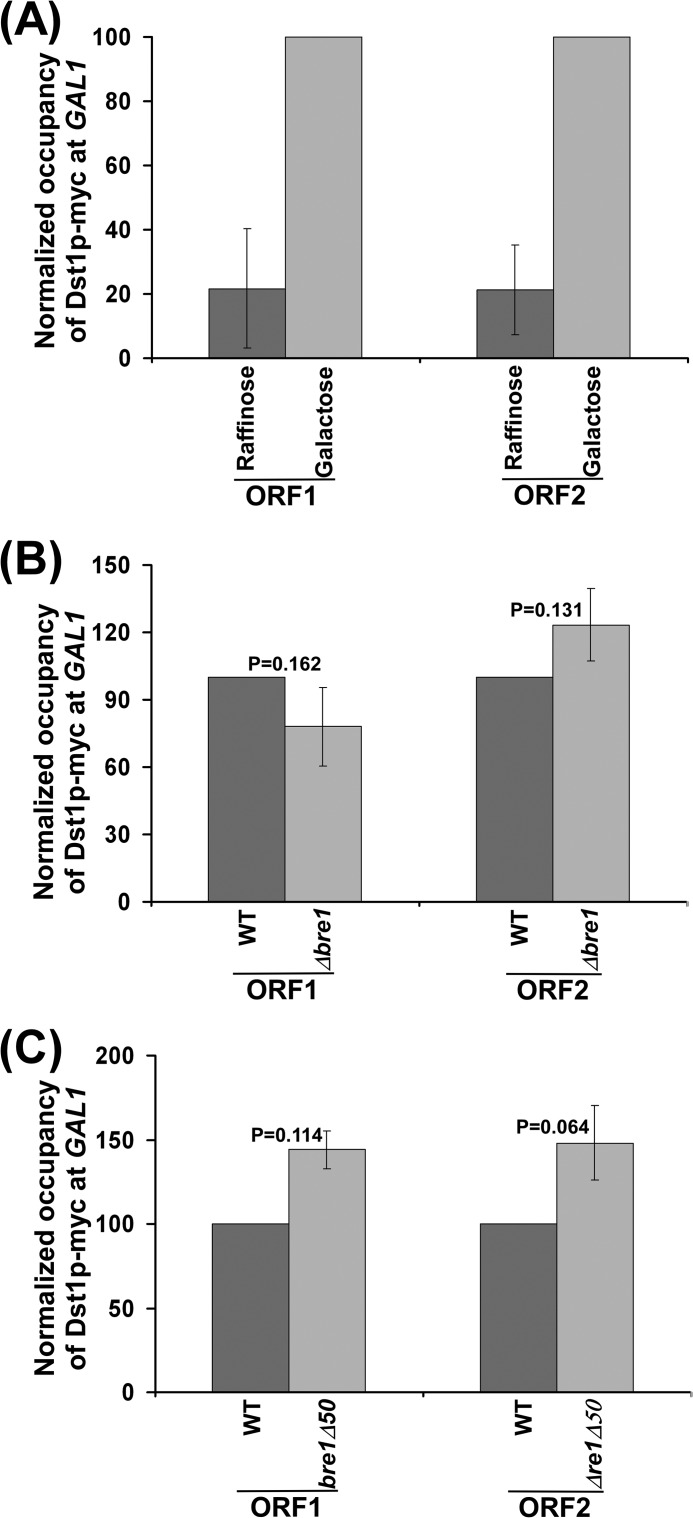
Association of TFIIS (Dst1p) with GAL1 is not altered in the absence of Bre1p or its RING domain. A, analysis of the association of TFIIS with GAL1 under inducible (galactose) and non-inducible (raffinose) conditions. Yeast strain expressing Myc-tagged Dst1p (RSY19) was grown at 30 °C in YPR up to an A600 of 0.9 prior to formaldehyde-based in vivo cross-linking. For GAL1 induction, a yeast strain (RSY19) was grown at 30 °C in YPR up to A600 of 0.9, and then transferred to YPG for 60 min prior to formaldehyde-based in vivo cross-linking. The ChIP assay was performed following the modified protocol as described under ”Experimental Procedures.“ Immunoprecipitation was carried out using an anti-Myc antibody (9E10; Santa Cruz Biotechnology, Inc.) against Myc-tagged Dst1p. The ChIP signals at ORF1 and ORF2 in galactose (or YPG) were set to 100. The ChIP signals at ORF1 and ORF2 in raffinose (or YPR) were normalized with respect to 100 (represented as normalized or relative occupancy). Error bars in YPR denote S.D. from three sets of biologically independent experiments. B, analysis of the association of TFIIS with GAL1 in the presence and absence of Bre1p. Both the wild-type and Δbre1 strains expressing Myc-tagged Dst1p (RSY19 and RSY20) were grown and cross-linked as described in the legend to Fig. 1B. Immunoprecipitation was performed as in panel A. C, analysis of the association of TFIIS with GAL1 in the presence and absence of the RING domain of Bre1p. Yeast cells were grown, cross-linked, and immunoprecipitated as in panel B.