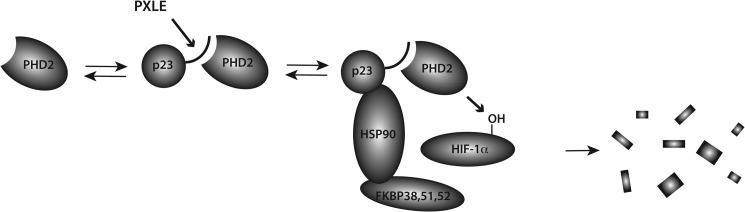
FIGURE 8.
Model for interaction of PHD2 with HSP90 pathway. PHD2 associates with p23 via a PXLE motif in p23 (denoted by curved line). p23 in turn is known to bind to the N terminus of HSP90. This facilitates PHD2-catalyzed hydroxylation of HIF-1α, which is known to be an HSP90 client protein. Prolyl hydroxylation in turn marks HIF-1α for degradation. The C terminus of HSP90 is known to bind a subset of HSP90 co-chaperones that includes FKBP38, FKBP51, and FKBP52. FKBP38 possesses a PXLE motif and, therefore, provides an independent means for recruiting PHD2 to the HSP90 machinery.