FIGURE 4.
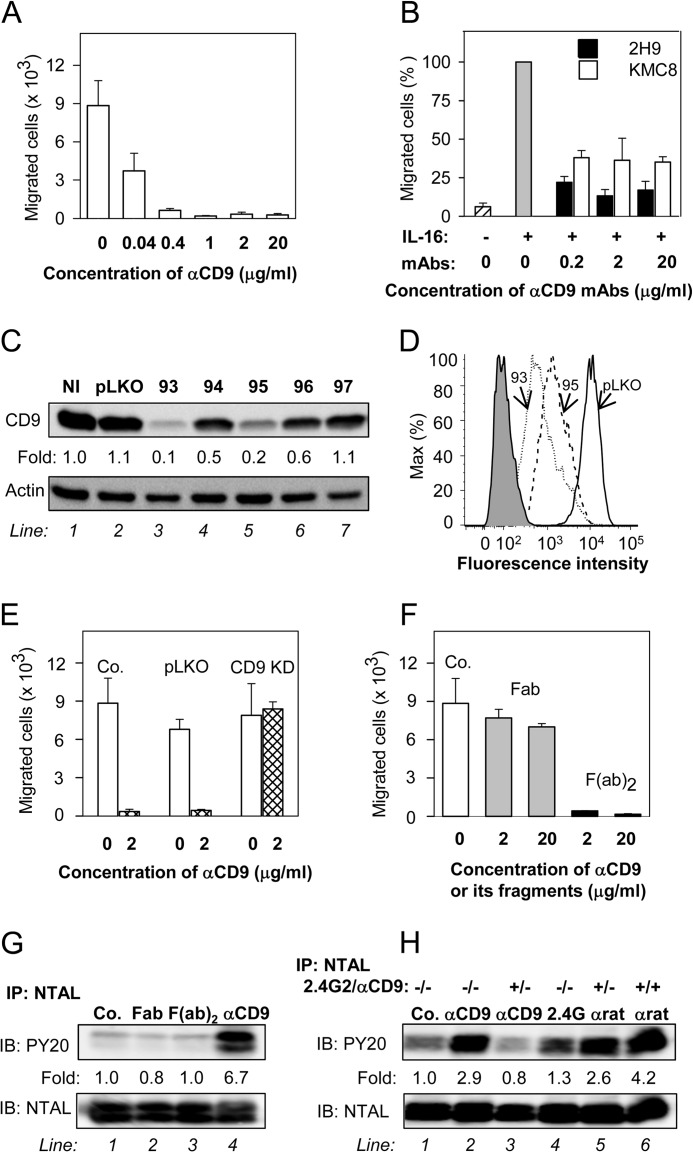
Anti-CD9 mAb inhibits chemotaxis toward Ag and induces tyrosine phosphorylation of NTAL by different mechanism. A, IgE-sensitized BMMCs were pretreated with the indicated concentrations of anti-CD9 mAb 2H9 for 15 min and their chemotactic response toward Ag (250 ng/ml of TNP-BSA in the lower chamber) was determined in the Transwell system. B, BMMCs were pretreated or not with the indicated concentrations of anti-CD9 antibodies (2H9 or KMC8) for 15 min and their chemotactic response toward IL-16 (50 ng/ml) was determined as above. Data were normalized toward the maximum response attained in the absence of antibody pretreatment. Migration in the absence of IL-16 is also shown. C and D, a set of murine CD9 shRNAs cloned into the pLKO.1 vector (TRCN0000066393 (93), TRCN0000066394 (94), TRCN0000066395 (95), TRCN0000066396 (96), TRCN0000066397 (97)) was used for lentiviral infection of BMMCs. After selection in puromycin, the cellular proteins were size fractionated by SDS-PAGE and analyzed by immunoblotting (IB) with anti-CD9 mAb 2H9. Actin was used as a loading control. Immunoblots were evaluated by densitometry and data were normalized to noninfected controls (NI) and actin amount. Similar results were obtained in at least three independent experiments. D, flow cytometry analysis of surface expression of CD9 in clones selected for further studies, 93 (dotted line) and 95 (dashed line). Gray filled region represents control cells exposed to secondary anti-rat Alexa 488 antibody alone. Thick line indicates cells infected with empty vector (pLKO). E, BMMCs were deprived of CD9 after infection with CD9 shRNA-containing vector (CD9 KD), uninfected cells (Co.), or cells infected with empty vector (pLKO) served as controls. Ag-mediated chemotaxis in the cells was measured as in A. F, BMMCs were not exposed (Co.) or exposed for 15 min to 2H9 mAb Fab or F(ab)2 fragments, each at a concentration 2 or 20 μg/ml. Their chemotaxis was determined as in A. G, BMMCs were exposed to BSSA (negative control, Co., line 1), 2H9 mAb Fab fragment (line 2), 2H9 mAb F(ab)2 fragment (line 3), or 2H9 whole molecule (αCD9; line 4); each at a concentration of 10 μg/ml. After 5 min the cells were solubilized in lysis buffer containing 1% Nonidet P-40 and 1% n-dodecyl-β-d-maltoside and postnuclear supernatants were immunoprecipitated (IP) with rabbit anti-NTAL antibody. The immunoprecipitates were analyzed by immunoblotting (IB) with phosphotyrosine-specific antibody PY-20-HRP conjugate (PY20) or NTAL-specific antibody as a loading control. Fold-increase in protein tyrosine phosphorylation, normalized to phosphorylation in nonactivated cells and NTAL amount is also indicated. A typical experiment from 4 performed is shown. H, BMMCs were pretreated or not with anti-CD16/CD32 (2.4G2; 1:50 diluted supernatant) and/or anti-CD9 mAb 2H9 (1 μg/ml, αCD9) for 15 min and then exposed to control BSSA (Co., line 1), anti-CD9 (1 μg/ml, lines 2 and 3), 2.4G2 antibody (1:50 diluted supernatant, line 4), or anti-rat IgG (1 μg/ml, lines 5 and 6). After 3 min the cells were lysed and NTAL was immunoprecipitated and analyzed as in G. Typical results from at least 3 experiments performed are shown. Mean ± S.D. in A, B, E, and F were calculated from 3 to 5 independent experiments.