FIGURE 5.
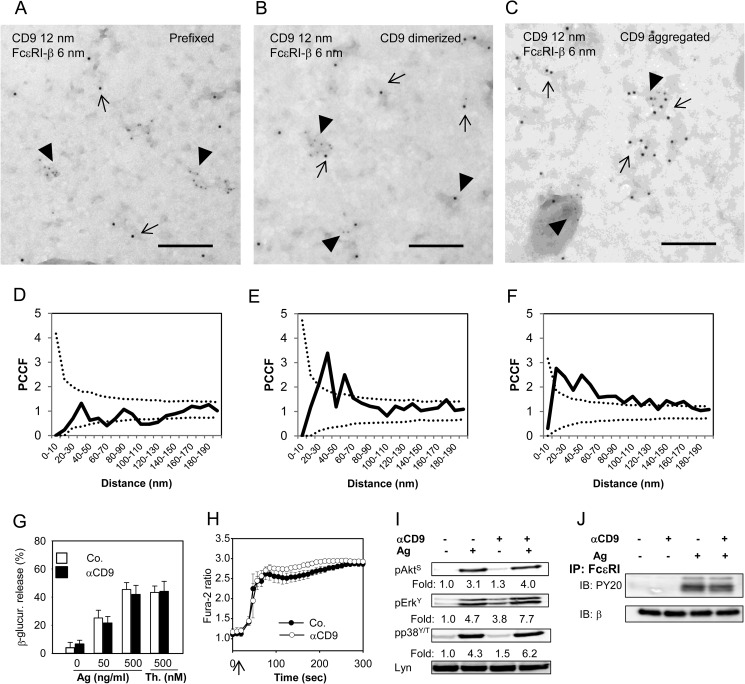
CD9 colocalizes with FcϵRI on the plasma membrane but CD9 aggregation does not interfere with early Ag-induced activation events. A and D, BMMCs derived from Balb/c mice were prefixed with paraformaldehyde and then labeled with anti-CD9 mAb 2H9 followed by secondary anti-rat antibody-12 nm gold conjugate. Plasma membrane sheets were then isolated and the FcϵRI-β subunit was labeled on the cytoplasmic side of the membrane with JRK mAb followed by secondary anti-mouse antibody-6 nm gold conjugate. Colocalization of CD9 (12 nm gold particles) and FcϵRI-β (6-nm gold particles) was analyzed by electron microscopy (A) and evaluated by PCCF (D) as described in the legend to Fig. 3. B and E, BMMCs were exposed to 2H9 mAb (CD9 dimerized) before fixation and labeling for CD9; other procedures and evaluations were as in A and D. C and F, the cells were exposed to 2H9 mAb followed by the secondary anti-mouse antibody (CD9 aggregation) and then fixed and further processed as in A and D. In A-C representatives from 3 independent experiments are shown. Bars, 200 nm. G-J, IgE-sensitized BMMCs derived from Balb/c mice were pretreated (αCD9) or not (Co.; −) with anti-CD9 mAb 2H9 (10 μg/ml) for 15 min before activation. G, the cells were exposed to various concentrations of Ag (0–500 ng/ml TNP-BSA) or 500 nm thapsigargin (Th.) and 30 min later amounts of β-glucuronidase released into the cell supernatants were determined. Mean ± S.D. were calculated from at least 3 independent experiments performed in triplicates. H, the cells were loaded with Fura-2AM at the time of exposure to anti-CD9 and stimulated (arrow) with Ag (500 ng/ml of TNP-BSA). [Ca2+]i was measured as described in the legend to Fig. 1B. Mean ± S.D. were calculated from 3 independent experiments performed in triplicates. I, IgE-sensitized BMMCs were exposed (+) or not (−) to anti-CD9 mAb 2H9 and then activated (+) or not (−) with Ag (100 ng/ml of TNP-BSA) for 3 min. Whole cell lysates were prepared and analyzed by immunoblotting with antibodies specific for pAkt-S473 (pAktS), pErk-Y204 (pErkY) or pp38-Y182/T180 (pp38Y/T); anti-Lyn mAb (Lyn) was used as a loading control. Fold-increase in protein phosphorylation, normalized to phosphorylation in nonactivated cells and protein loading is also shown. Typical results from at least 4 experiments performed are shown. J, IgE-sensitized BMMCs were exposed (+) or not (−) to anti-CD9 mAb and then activated by Ag (+; 250 ng/ml of TNP-BSA) or not (−). After 5 min the cells (15 × 106 per sample) were solubilized in lysis buffer containing 0.2% Triton X-100 and FcϵRI was immunoprecipitated from postnuclear supernatants by anti-IgE antibody immobilized to Protein A beads. Tyrosine phosphorylation of the receptor subunits was evaluated with PY-20-HRP conjugate (PY-20). The amount of immunoprecipitated receptor was estimated by immunoblotting (after stripping of the membrane) with JRK mAb recognizing FcϵRI β subunit. A typical experiment from 3 performed is shown.