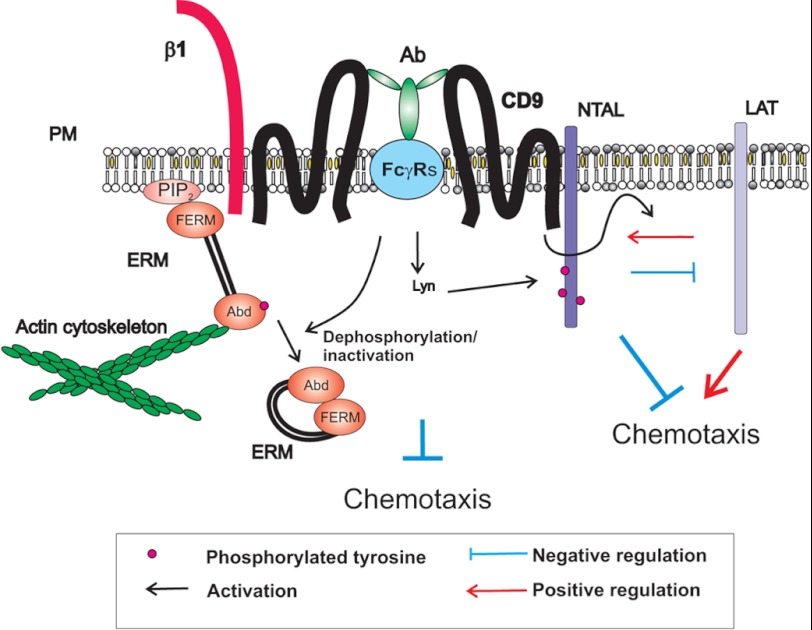
FIGURE 8.
A model of chemotaxis regulators involving CD9 and NTAL-LAT cross-talk. Tetraspanin CD9 resides in the plasma membrane (PM) in close proximity to β1-integrin (β1) and NTAL. CD9-specific antibody 2H9 (Ab) binds to CD9 and FcγR through its Ag-binding site and Fc region, respectively. This leads to tyrosine phosphorylation of NTAL and other proteins. CD9 cross-linking also results in dephosphorylation of the regulatory threonine of ERM family proteins leading to changes in their conformation and subsequent disconnection from binding to phosphatidylinositol 4,5-bisphosphate (PIP2) and/or other membrane components (throughout N-terminal FERM domain) and actin cytoskeleton (through actin-binding domain, Abd). These and other events under certain conditions inhibit mast cell chemotaxis. Chemotaxis is also regulated by cross-talk between NTAL, LAT, and CD9. LAT and NTAL seem to be, respectively, predominantly positive and negative regulators of chemotaxis. Binding of the anti-CD9 mAb 2H9 interferes with NTAL-LAT cross-talk.