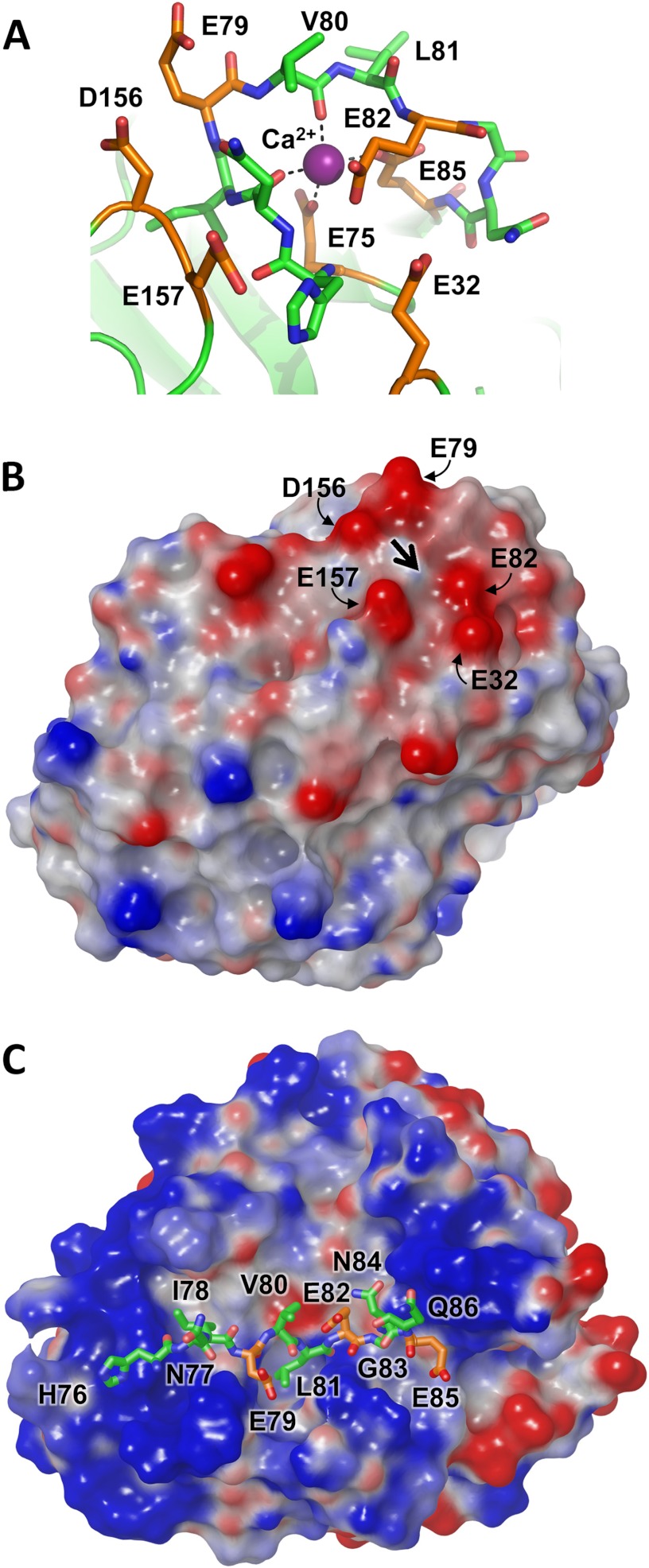
FIGURE 4.
Cationic trypsinogen Ca2+-binding loop targeted for cleavage by CTRC. A, the Ca2+-binding loop of human cationic trypsinogen contains multiple acidic residues (orange), with additional acidic residues located in nearby neighboring loops. The bound Ca2+ is shown in purple; the Leu81-Glu82 peptide bond represents the preferred cleavage site targeted by CTRC. Structure coordinates are from PDB code 2RA3 (55). B, the electrostatic surface potential calculated for cationic trypsin reveals a negative charge surrounding the site of bound Ca2+, indicated by the large black arrow. Molecular surfaces were generated using the Molecular Surface module of Schrodinger 2012 as described under “Experimental Procedures”; the electrostatic potential color ramp was set from a minimum of −0.35 to a maximum of 0.15. C, Ca2+-binding loop cleavage sequence of cationic trypsinogen is shown modeled into the active site cleft of CTRC. The hydrophobic side chains of Ile78 and Leu81 occupy the complementary S4 and S1 subsites, respectively. Acidic residues are shown in orange; Glu79 at the P2 position and Glu85 at the P4′ position are near CTRC regions of positive surface potential.