Figure 1.
ACO2 Is Succinated on Critical Cysteine Residues in Fh1KO MEFs
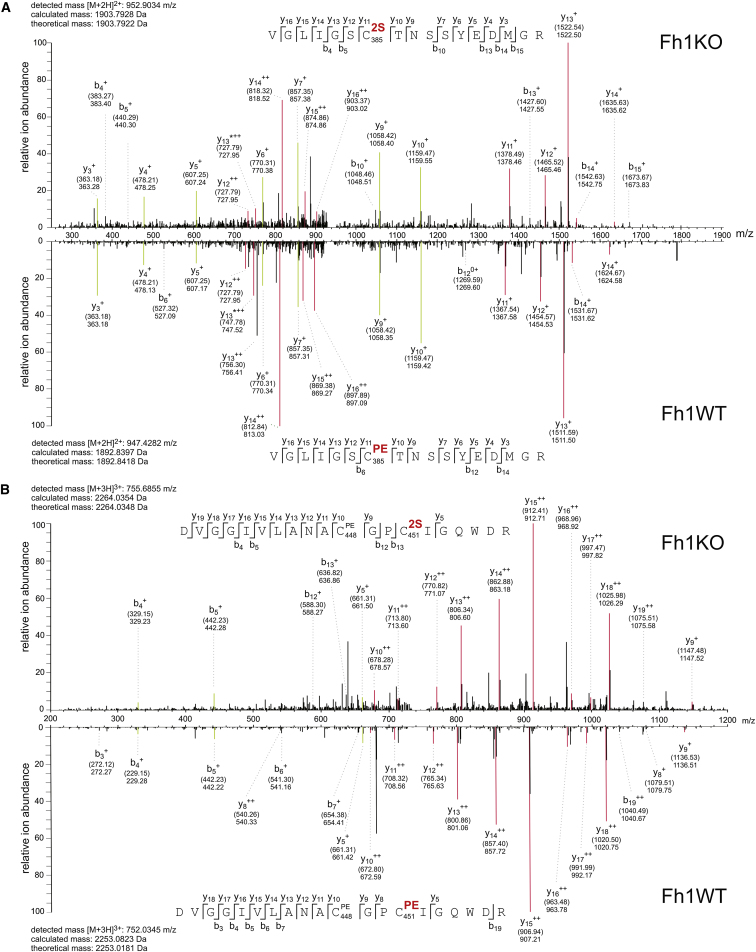
(A and B) MS/MS spectra showing succination at C385 in the 379-VGLIGS(2SC)TNSSYEDMGR-395 peptide (A) and at C451 in the 438-DVGGIVLANA(PEC)GP(2SC)IGQWDR-457 peptide (B) (upper panels) derived from endogenous ACO2 in Fh1KO MEFs. Spectra are shown in direct comparison with the originally unmodified counterpeptides that are pyridylethylated on the corresponding cysteines detected in Fh1WT cells (lower panel). Selected fragments were assigned as follows: b, N-terminal fragment ion; y, C-terminal fragment ion; ∗, fragment ion minus NH3; 0, fragment ion minus H2O; +, singly charged fragment ion; ++, doubly charged fragment ion; PE, pyridylethylated; 2S, succinated. Both theoretical mass (in brackets) and detected mass are given for each assigned fragment ion. Fragment ion mass signals that were assigned for both peptide species and contain the modified cysteine residue are highlighted in red, whereas fragments that do not comprise the modification are highlighted in green. Note that for fragment ions that include the modified cysteine, singly charged fragment ions are shifted according to the mass difference between 2S (116.01 Da) and PE (105.06) modifications by 10.95 Da, whereas doubly charged fragment signals are shifted by 5.48 Da.
See also Figure S1.