Figure 4.
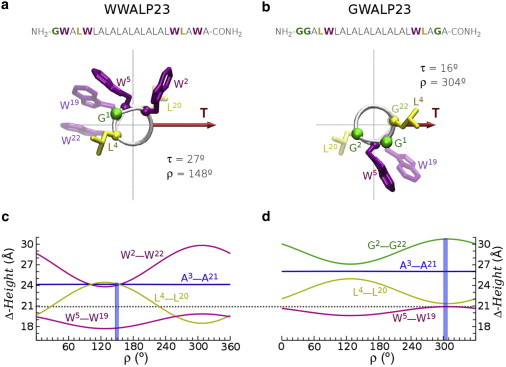
Sequences and structure models of helical peptides WWALP23 (a) and GWALP23 (b). The helices are viewed from the N-terminus along their long axis, such that the tilt vector (T) is pointing to the right and has a length proportional to the experimental τ angle (values corresponding to DLPC membranes, determined in (6) using 2H NMR splittings from (17)). The azimuthal rotation ρ, also from experiments (6,17), is defined relative to the radial position of the Cα of residue G1. The side chains of the flanking tryptophans (W2, W22, W5, and W19) and relevant hydrophobic residues (L4, L20) are represented by sticks, in arbitrary conformation. Underneath are shown the hydrophobic and flanking Δ-heights for relevant residues (labeled in the graphs) of WWALP23 (c) and GWALP23 (d) as functions of ρ for their respective experimental τ. The experimental values of ρ are marked in the plots with vertical bars. The horizontal dotted line corresponds to the estimated hydrophobic thickness of liquid crystalline DLPC (20.9 Å (28)).
