Figure 5.
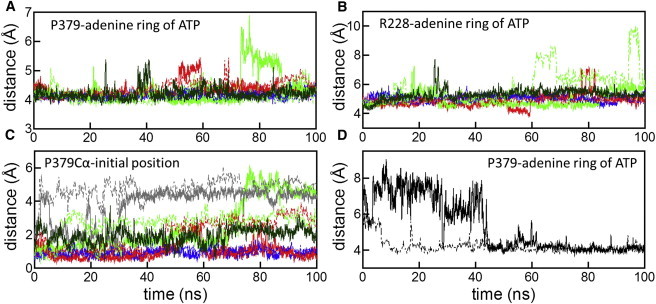
The adenine ring of ATP mediates interactions between adjacent protomers. In panel A, distances between the centers of mass of the P379 side chain (protomer B) and of the adenine ring of ATP are shown. In panel B, distances between the centers of mass of the R228 side chain (protomer A) and of the adenine ring of ATP are shown. In panel C, displacements of the Cα of P379 (protomer B) from the initial position during simulations are shown. In panels A–C, the colors of the lines indicate simulations K2NATPIM (blue), K1NATPIM (red), K0NATPIM (green), and K2N-IM (gray). In panel D, in simulation K2NATPIC starting from the crystal structure, distances between the centers of mass of the P379 side chain (protomer B) and of the adenine ring of ATP are shown. The spontaneous binding of P379 to the adenine ring was observed. Solid and dashed lines represent the results of the first and second runs, respectively. The third run of simulation K0NATPIM is indicated by solid dark green lines.
