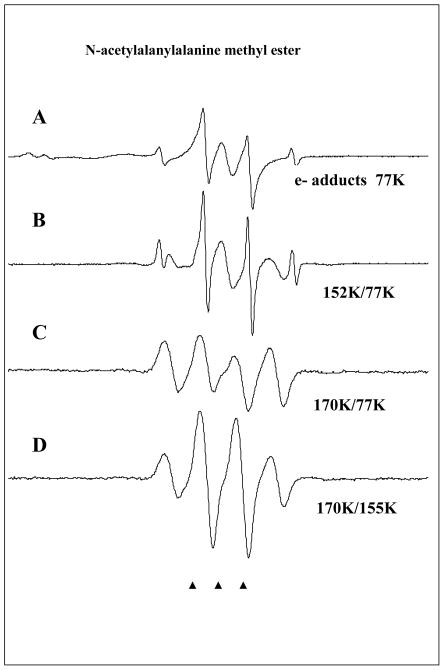
Figure 4.
ESR spectra found after electron attachment to N-acetylalanylalanine methyl ester at 77K and annealing to temperatures shown in the figure. A. Spectrum at 77K immediately after gamma irradiation. The ESR spectrum consists of radicals formed by electron attachment to the three possible sites in the structure and Cl2−•. The strong signal from the methyl radical is a result of C-O bond cleavage at 77K induced by electron attachment. The doublet which broadens the central two CH3• radical lines is from electron attachment to the N-acetyl portion. The central singlet is assigned to electron attachment at the alanyl and ester sites. B. On warming the methyl radical increases and main chain cleavage occurs to form the alanyl radical, •CHCH3C(O)R, which shows a quintet from couplings to four nearly equivalent protons (24 G avg each). C. On annealing to 170 K all radicals abstract from the alpha hydrogens on the peptide backbone to produce the –NH-C(•)(CH3)-C(O)- radical with an 18 G coupling from 3 equivalent methyl group protons showing hindered rotation at 77K. All spectra in A–C are recorded at 77K after annealing to temperatures in the figure. D. This spectrum was recorded at 155K and shows the expected pattern from a methyl group in near free rotation. The change in spectra from C to D was reversible with temperature and is only a result of rotation of the methyl group.