Abstract
The aim of this study was to examine infant feeding and the long‐chain polyunsaturated fatty acid (LCPUFA) concentration of breast milk and formulas in relation to infant development. The prospective Pregnancy, Infection and Nutrition Study (n = 358) collected data on breastfeeding, breast milk samples and the formulas fed through 4 months post‐partum. At 12 months of age, infants' development was assessed (Mullen Scales of Early Learning). Linear regression was used to examine development in relation to breastfeeding, breast milk docosahexaenoic acid (DHA) and arachidonic acid (AA) concentration, and DHA and AA concentration from the combination of breast milk and formula. The median breast milk DHA concentration was 0.20% of total fatty acids [interquartile range (IQR) = 0.14, 0.34]; median AA concentration was 0.52% (IQR = 0.44, 0.63). Upon adjustment for preterm birth, sex, smoking, race and ethnicity and education, breastfeeding exclusivity was unrelated to development. Among infants exclusively breastfed, breast milk LCPUFA concentration was not associated with development (Mullen composite, DHA: adjusted β = −1.3, 95% confidence interval: −10.3, 7.7). Variables combining DHA and AA concentrations from breast milk and formula, weighted by their contribution to diet, were unassociated with development. We found no evidence of enhanced infant development related to the LCPUFA content of breast milk or formula consumed during the first four post‐natal months.
Keywords: arachidonic acid, breast milk, docosahexaenoic acid, infant feeding, polyunsaturated fatty acids, breastfeeding.
Introduction
The role of long‐chain polyunsaturated fatty acids (LCPUFAs) in brain development has long been of interest. Fatty acids are important constituents of cell membranes, and docosahexaenoic acid (DHA) in particular accumulates in high concentration in the developing brain (Kishimoto et al. 1969, Sun & Sun 1972). DHA and other LCPUFAs like arachidonic acid (AA) are available to infants through breast milk and supplemented formulas.
LCPUFA supplementation seems to accelerate some developmental domains like visual acuity and general mental development among preterm infants (Carlson et al. 1996; Fewtrell et al. 2004; Clandinin et al. 2005; Makrides et al. 2009), but the degree to which LCPUFAs are associated with other aspects of development like language or motor development and among term infants is less clear (Scott et al. 1998; Makrides et al. 2000; Auestad et al. 2001; O'Connor et al. 2001; Fewtrell et al. 2002). Most studies have been randomized trials, often of preterm infants, and focused on general mental or motor development or early visual acuity rather than later‐developing components of language or visual reception. A randomized design can be extremely advantageous, but studies comparing infants fed supplemented formulas with breastfed infants present a problem if they do not effectively control for factors associated with the choice to breastfeed vs. formula feed because such factors also influence development. Most trials supplementing mother's milk use an intent‐to‐treat analysis, comparing groups rather than the LCPUFAs concentration reaching individuals; thus, they do not consider variability in exposure from maternal or infant diet outside the supplement. Most US children are introduced to formula during infancy, and by 6 months of age, only 13% are exclusively breastfed (CDC 2010). Both breast milk and infant formulas can be significant sources of LCPUFAs, but the amount contributed may vary considerably according to feeding method (breast vs. formula) and composition of formula. Furthermore, studies of complex feeding patterns observed in the general population that distinguish the psychosocial contribution of breastfeeding from breast milk and formula LCPUFAs are lacking in the literature.
We address three questions. First, is the extent of breastfeeding during the first four post‐natal months associated with visual reception, receptive and expressive language, motor skills and overall cognitive development at 12 months of age? Second, among exclusively breastfed infants, is the LCPUFA concentration of breast milk associated with development? Third, is the LCPUFA concentration over the first four post‐natal months (derived from individual combinations of breast milk and formula LCPUFA concentrations) associated with development?
Key messages
-
•
Infants who were exclusively or almost exclusively breastfed for the first 4 months exhibited more advanced development at 12 months than exclusively formula‐fed infants, but this association was attenuated after controlling for key confounders.
-
•
The docosahexaenoic acid (DHA) content of breast milk, or breast milk and formula combined, was not associated with infant development.
-
•
Future studies should consider individual exposures using the actual concentrations of fatty acids infants are exposed to, as a complement to analyses that compare treatment groups, to better clarify whether there are benefits of DHA supplementation to infants born at term.
Materials and methods
Study population
Data were from the Pregnancy, Infection and Nutrition (PIN) Study and its post‐natal follow‐up components, PIN Postpartum and PIN Babies. The PIN Study assessed the association of prenatal nutrition, lifestyle and infection with preterm birth using data from medical records, interviews and specimens provided during pregnancy (Savitz et al. 1999). Women were recruited from among those attending prenatal clinics at less than 20 weeks of pregnancy at the University of North Carolina Hospitals between January 2001 and June 2005. Women were ineligible if they were pregnant with multiple fetuses, could not communicate in English, were under the age of 16 years, had no access to a telephone or were intending to go elsewhere for future care or delivery. The PIN Postpartum Study assessed behaviours associated with high gestational weight gain and post‐partum weight retention through data collected during home interviews around 4 and 12 months post‐partum (Evenson et al. 2009). Women (n = 1 169) were eligible for PIN Postpartum if they completed the PIN Study, agreed to be contacted after delivery and lived in the study area. Medical constraints (n = 24), inability to re‐contact (n = 207) or schedule (n = 62) and refusal (n = 187) resulted in 689 women who participated in a visit scheduled in the fourth post‐partum month. Before the 12‐month data collection visit, 45 became pregnant again and were ineligible to continue, 62 were unreachable, 29 moved from the study area and 20 requested to leave the study. The PIN Babies Study assessed the association between the prenatal and early post‐natal environment and child development. Infants' development was assessed during the PIN Postpartum home visits. In January 2004, infants of participating women became eligible for the PIN Babies protocol. Among the remaining 408 eligible maternal–infant pairs, some did not participate in the Mullen developmental assessment because the child was not present during the data collection (n = 11, e.g. the mother completed the interview at her workplace), the child was asleep, sick or fussy (n = 21), the mother refused the child's participation (n = 8), there was not enough time during the visit (n = 3) or various other reasons (n = 7). The university's institutional review board approved all protocols; all participants provided written informed consent. Infants who completed at least part of the Mullen at 12 months were included in this analysis.
Exposure measurement
At the home visit at 4 months post‐partum, women provided information about the number of times per day the infant was breastfed or fed formula or other foods for each preceding month. Women who were still breastfeeding were asked to use a breast pump at around 10 am on the day of the visit to provide three 1.5‐mL tubes of milk for storage at −80°C. Samples collected before April 2005 were analysed by the Collaborative Studies Clinical Laboratory at the University of Minnesota Medical Center, Fairview (Minneapolis, MN, USA). This laboratory was unable to analyse a second batch, so samples collected later were analysed at the Clinical Nutrition Research Center of the University of North Carolina (Chapel Hill, NC, USA). Fatty acid extraction used the method of Bligh & Dyer (1959). The individual fatty acids were identified by comparison with authentic standards (Nu Chek Prep, Elysian, MN, USA). Data were analysed using Perkin Elmer Totalchrom Chromatography Software, version 6.2 (Somerset, NJ, USA).
Feeding method was classified for each month of the first 4 months. Although feeding methods could change within a particular month, women were asked to consider the predominant method for the month. Infants who were breastfed for all feedings per day were considered exclusively breastfed. Infants who were breastfed and also had one formula feeding per day were considered almost exclusively breastfed. Infants who were fed formula for all daily feedings were considered exclusively formula fed. All others were considered partially breastfed. For the 4‐month period as a whole, infants who were exclusively or almost exclusively breastfed all months or exclusively formula fed all months were classified accordingly. All others were classified as partially breastfed for the 4‐month period as a whole.
For each month of exclusive or almost exclusive breastfeeding, breast milk LCPUFA concentrations were assigned as the exposure value. For each month of formula feeding, the names of up to two formulas were recorded. The fatty acid concentration for each formula was assigned using data from the US Department of Agriculture Nutrient Database for Standard Reference (US Department of Agriculture 2008). If more than one formula was fed in a month, the mean value for the two most fed was used.
We created a variable reflecting the combination of DHA and AA concentrations in breast milk and USDA‐based concentrations of DHA and AA in the formula(s) for each month. To account for variation in feeding method across months, the composite variable was weighted by the relative contribution of breast milk and formula for each month. Each month of partial breastfeeding was assigned half the laboratory value for the breast milk plus half the average formula value for that month. DHA and AA exposure variables for all 4 months combined were calculated by summing the monthly values:
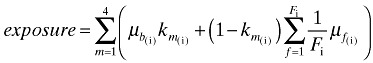 |
where m = month since birth, µ = concentration of the fatty acid of interest in breast milk (b) or infant formula (f) (units: % of total fatty acids), k = feeding pattern in m (1, if exclusive or almost exclusive breastfeeding; 0.5, if partial breastfeeding; 0, if formula feeding), F = number of formulae fed (F i ≤ 2) and f = formula product.
Outcome measurement
The Mullen was administered by four trained staff in the home when infants reached 12 months of age {inter‐rater reliability = 0.83 [95% confidence interval (CI): 0.70, 0.93] } (Mullen 1995). The Mullen assesses cognitive functioning on five subscales: visual reception, fine motor, receptive language, expressive language and gross motor. Raw scores were converted to age‐specific t‐scores and four subscales (all but gross motor) were summed per the manual to create the Early Learning Composite Score (composite) (Mullen 1995). Examples of the abilities assessed for this age include standing, looking for objects, stacking blocks, understanding gestures with commands and jabbering with inflection. Scores were not adjusted for gestational age at birth because the procedure recommended in the Mullen manual overcorrected scores for preterm infants. However, an indicator of preterm status was included in models.
Covariate measurement
Data on maternal age, education and other demographic and lifestyle covariates were obtained via telephone in prenatal weeks 17–22. Around 20 weeks gestation, women completed the State‐Trait Anxiety Inventory (Spielberger 1983). Parity was defined as the number of prior live or stillbirths. Gestational age was based on ultrasound if done before 22 weeks or the date of the last menstrual period from the hospital record. We defined preterm birth as less than 37 completed weeks' gestation. At the first post‐natal visit, women reported current smoking and family income and completed the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al. 1987).
Statistical analysis
We built three sets of linear regression models: (1) Breastfeeding models: compared with exclusively formula‐fed infants, we assessed whether the degree of breastfeeding over the first four post‐partum months (exclusive/almost exclusive, partial or formula feeding) was associated with Mullen subscale and composite scores; (2) Breast milk models: among infants exclusively breast fed through the first 4 months, we examined the association between the breast milk DHA and AA concentration and Mullen scores; and (3) Combined models: we evaluated total DHA and AA represented by the constructed variables that incorporated the proportional concentrations of DHA and AA from both formula and breast milk sources in relation to Mullen scores. For the breast milk and combined models, we also examined the DHA : AA ratio.
Possible covariates were identified a priori based on previous studies, and each was evaluated for inclusion in adjusted models by selecting those that changed the exposure variable beta coefficient by >10% when removed from a model. We evaluated income (<185, 185–350, >350% of federal poverty level), education (≤high school, >high school), maternal age (16–20, 21–30, 31+ years), parity (0, 1+), race and ethnicity (white non‐Hispanic, all others), sex, preterm birth (<37 weeks gestation), trait anxiety (tertiles) and depressive symptoms (EPDS 0–9 vs. 10+). Wilcoxon rank‐sum, chi‐square and t‐tests were used to examine differences in exposure and outcome distributions across levels of covariates. Multiple partial F‐tests assessed the presence of effect modification. All models that examined breast milk included a variable indicating the laboratory that analysed the sample, a statistical technique for adjusting for any differences between laboratories. SAS 9.1 (SAS Institute, Cary, NC, USA) was used for analyses.
Results
Eighty‐nine per cent of women in this sample breastfed. The proportions of infants exclusively or almost exclusively breastfed, partially breastfed and exclusively formula fed are shown in Fig. 1a. By the time of breast milk collection, 68 of the 358 eligible women had discontinued breastfeeding, 231 provided a sample (230 were analyzable), 17 were breastfeeding but unable to provide a sample, three refused milk collection and 39 had exclusively formula fed their infant. DHA in samples ranged from 0.07% to 1.49% of total fatty acids [mean = 0.28%, standard deviation (SD) = 0.22] (Table 1). AA ranged from 0.05% to 1.52% of total fatty acids (mean 0.57%, SD = 0.20).
Figure 1.
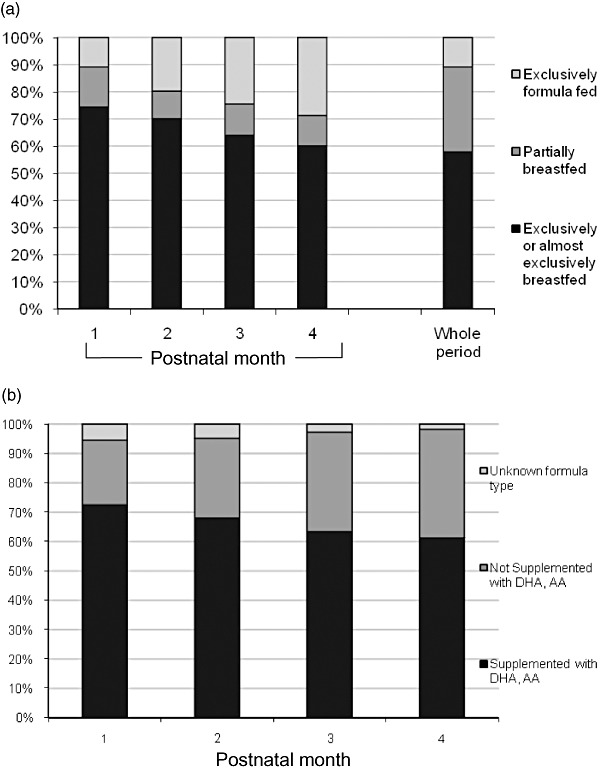
(a) The proportion of infants exclusively or almost exclusively breastfed, partially breastfed and exclusively formula fed in each of the first four post‐natal months and all 4 months combined. For the whole period, the partially breastfed group consists of infants who were at least partially breastfed in at least 1 month but not exclusively breastfed for all 4 months. PIN 2001–2006 [from PIN (1995–2006)] Babies Study. (b) The proportion of formulas supplemented with docosahexaenoic acid (DHA) and arachidonic acid (AA) over the first four post‐natal months fed to infants not exclusively breastfed. PIN 2001–2006 [from PIN (1995–2006)] Babies Study.
Table 1.
Breast milk DHA and AA concentrations, feeding patterns and characteristics of infants in the PIN 2001–2006 [from PIN (1995–2006)] Babies Study
| (n, %) | Breast milk concentration (n = 231) | Feeding method (first four post‐natal months)* | Mullen composite (mean, SD) | ||||
|---|---|---|---|---|---|---|---|
| Median DHA% of total fatty acids (IQR) † | Median AA% of total fatty acids (IQR) † | Exclusively or almost exclusively breastfed (n, %) | Partially breastfed (n, %) | Exclusively formula fed (n, %) | |||
| Overall | 358 (100.0) | 0.20 (0.14, 0.34) | 0.52 (0.44, 0.63) | 207 (57.8) | 112 (31.3) | 39 (10.9) | 99.4 (13.6) |
| Race and ethnicity | |||||||
| White, non‐Hispanic | 283 (79.1) | 0.22 (0.14, 0.34) | 0.52 (0.45, 0.63) | 184 (65.0) | 77 (27.2) | 22 (7.8) ‡ | 100.1 (13.6) |
| All others | 75 (21.0) | 0.16 (0.13, 0.37) | 0.49 (0.43, 0.62) | 23 (30.7) | 35 (46.7) | 17 (22.7) | 97.1 (13.8) |
| Maternal education | |||||||
| High school or less | 50 (14.0) | 0.18 (0.12, 0.23) | 0.53 (0.42, 0.62) | 7 (14.0) | 29 (58.0) | 14 (28.0) ‡ | 95.7 (14.7) § |
| Greater than high school | 308 (86.0) | 0.20 (0.14, 0.36) | 0.52 (0.44, 0.63) | 200 (64.9) | 83 (27.0) | 25 (8.1) | 100.1 (13.4) |
| Smoking | |||||||
| Yes | 44 (12.3) | 0.30 (0.21, 0.42) | 0.53 (0.44, 0.73) | 10 (22.7) | 23 (52.3) | 11 (25.0) ‡ | 94.3 (12.6) § |
| No | 314 (87.7) | 0.19 (0.14, 0.33) | 0.52 (0.45, 0.62) | 197 (62.7) | 89 (28.3) | 28 (8.9) | 100.1 (13.6) |
| Parity | |||||||
| 0 | 186 (52.0) | 0.21 (0.16, 0.38) ¶ | 0.52 (0.44, 0.65) | 104 (60.5) | 55 (32.0) | 13 (7.6) | 99.6 (13.9) |
| ≥1 | 172 (48.0) | 0.19 (0.12, 0.31) | 0.52 (0.45, 0.62) | 103 (55.4) | 57 (30.7) | 26 (14.0) | 99.3 (13.4) |
| Infant sex | |||||||
| Female | 165 (46.1) | 0.20 (0.14, 0.42) | 0.53 (0.4, 0.636) | 94 (57.3) | 54 (32.9) | 16 (9.8) | 102.3 (14.0) § |
| Male | 193 (53.9) | 0.19 (0.14, 0.30) | 0.50 (0.43, 0.62) | 113 (58.6) | 57 (29.5) | 23 (11.9) | 97.1 (12.8) |
| Preterm birth | |||||||
| Preterm | 45 (12.6) | 0.17 (0.12, 0.24) | 0.49 (0.46, 0.63) | 14 (31.1) | 22 (48.9) | 9 (20.0) ‡ | 94.1 (14.8) § |
| Term | 313 (87.4) | 0.20 (0.14, 0.36) | 0.52 (0.44, 0.63) | 193 (61.7) | 90 (28.8) | 30 (9.6) | 100.2 (13.3) |
| Laboratory | |||||||
| Minnesota | 128 (55.4) | 0.16 (0.12, 0.22) ¶ | 0.49 (0.43, 0.55) ¶ | 109 (85.2) | 19 (14.8) | – | 100.6 (14.2) |
| North Carolina | 103 (44.6) | 0.31 (0.19, 0.46) | 0.60 (0.47, 0.79) | 86 (83.5) | 17 (16.5) | – | 101.7 (13.0) |
AA, arachidonic acid; IQR, interquartile range; DHA, docosahexaenoic acid; SD, standard deviation. *Infants who were breastfed for all feedings per day for the first four post‐natal months were considered exclusively breastfed. Infants who were breastfed plus up to one formula feeding per day were considered almost exclusively breastfed. Infants who were fed infant formula for all daily feedings were considered exclusively formula fed. All other infants were partially breastfed. †Interquartile range. ‡ χ 2 P < 0.05. § t‐test P < 0.05. ¶Wilcoxon rank‐sum test P ≤ 0.05.
In general, women who exclusively or almost exclusively breastfed were more likely to be white, non‐Hispanic, non‐smokers, well‐educated and have term infants than those who partially or exclusively formula fed. There were few differences in the LCPUFA distributions across the covariates, but DHA concentration varied by parity and laboratory. No participant characteristics or outcomes were associated with which laboratory analysed the samples (data not shown).
Among infants receiving some formula, the proportion that received LCPUFA‐supplemented formulas each month is shown in Fig. 1b. Only eight were fed more than one formula in any month, and three were fed non‐supplemented formulas for all 4 months. Six women could not identify the formula they fed and were excluded from the combined models. The fatty acid concentration of formulas ranged from 0.30% to 0.37% for DHA and from 0.50% to 0.67% for AA. Feedings of other foods were uncommon in this cohort until 3 months of age when the most common were cereal (14% were fed cereal one or more times per day), fruit or vegetables (4%) and juice (4%).
Mullen scores were as expected for a general population sample of infants (mean subscale t‐scores ∼50, mean composite score = 99). Scores were higher among females (t = −3.5, P < 0.01) and term (t = −2.8, P < 0.01) infants and those whose mothers were more educated (t = −2.1, P = 0.04) and non‐smokers (t = 2.6, P = 0.01). Scores among children whose mothers were still breastfeeding at the time of milk collection but did not provide a milk sample were not notably different from those who provided samples (t = 0.6, P = 0.54). Children whose mothers breastfed but had quit by the time of milk collection scored lower (mean composite = 96.3, SD = 13.1) than children whose mothers were still breastfeeding (mean composite = 101.1, SD = 13.7) (t = 2.5, P = 0.01).
In unadjusted models, infants who were exclusively or almost exclusively breastfed for the first 4 months exhibited more advanced development in several domains compared with infants who were formula fed (e.g. unadjusted β for composite = 6.2, 95% CI: 1.6, 10.8). However, these differences were consistently attenuated (adjusted effect estimates for all subscales were within 3 points of null) upon adjustment for confounders and were no longer statistically significant (α = 0.05) (e.g. adjusted β for composite = 3.7, 95% CI: −1.3, 8.6) (Table 2). Thus, we conclude that feeding method was not associated with development once key confounders were included in the analysis.
Table 2.
Results from linear regression models for the association between feeding method for the first four post‐natal months and Mullen Scales of Early Learning scores, PIN 2001–2006 [from PIN (1995–2006)] Babies Study
| Mullen Scales of Early Learning | Feeding method* (exclusively formula fed = reference, n = 39) | |||
|---|---|---|---|---|
| Exclusively or almost exclusively breastfed (n = 207) | Partially breastfed (n = 112) | |||
| β (CI) (unadjusted) | β (CI) (adjusted) † | β (CI) (unadjusted) | β (CI) (adjusted) † | |
| Gross motor | 1.7 (−2.4, 5.8) | 0.9 (−3.5, 5.3) | 0.9 (−3.5, 5.3) | 0.6 (−3.7, 4.9) |
| Visual reception | 4.4 (0.7, 8.2) | 2.4 (−1.7, 6.5) | 0.6 (−3.4, 4.7) | −0.2 (−4.2, 3.9) |
| Fine motor | 4.6 (0.8, 8.3) | 2.5 (−1.6, 6.5) | 2.2 (−1.8, 6.3) | 1.1 (−2.9, 5.1) |
| Receptive language | 1.2 (−1.6, 3.9) | 0.5 (−2.4, 3.5) | −1.2 (−4.1, 1.7) | −1.5 (−4.4, 1.4) |
| Expressive language | 2.2 (−0.9, 5.3) | 1.5 (−1.8, 4.9) | 0.4 (−2.9, 3.7) | 0.1 (−3.3, 3.3) |
| Composite | 6.2 (1.6, 10.8) | 3.7 (−1.3, 8.6) | 1.0 (−4.0, 5.9) | −0.3 (−5.2, 4.6) |
CI, confidence interval. *Infants who were breastfed for all feedings per day for the first four post‐natal months were considered exclusively breastfed. Infants who were breastfed plus up to one formula feeding per day were considered almost exclusively breastfed. Infants who were fed infant formula for all daily feedings were considered exclusively formula fed. All other infants were partially breastfed. †Adjusted models include education, race and ethnicity, smoking, infant sex and preterm status.
Among infants exclusively breastfed, breast milk DHA concentration was not associated with Mullen scores after adjustment for sex, parity, smoking, preterm status and laboratory – almost all adjusted beta coefficients were within 3 points of null and all CI included the null (e.g. adjusted β for composite = −1.3, 95% CI: −10.3, 7.7) (Table 3). Although AA concentration was positively associated with development in unadjusted models, especially gross motor development (beta coefficients were >3 points from the null for all subscales), the estimates were very imprecise and almost all CI included the null. Once confounders were considered, almost all of the effect estimates were slightly attenuated and all effect estimates were not statistically significant (α = 0.05) (e.g. adjusted β for gross motor = 9.0, 95% CI: −1.0, 19.0). Results for the DHA : AA ratio for all scales did not differ from the null (results not shown). As a result, we conclude that breast milk DHA content was not associated with cognitive development because of the small magnitude of the effect estimates (for DHA) and the lack of statistical significance (for DHA and AA).
Table 3.
Results from linear regression models for the association between docosahexaenoic acid and arachidonic acid in breast milk and Mullen Scales of Early Learning scores among infants exclusively breastfed (n = 183) for the first four post‐natal months, PIN 2001–2006 [from PIN (1995–2006)] Babies Study
| Mullen Scales of Early Learning | Breast milk DHA (continuous) | Breast milk AA (continuous) | ||
|---|---|---|---|---|
| β (CI) (unadjusted)*, † | β (CI) (adjusted) ‡ | β (CI) (unadjusted)*, † | β (CI) (adjusted) ‡ | |
| Gross motor | 5.6 (−2.8, 13.9) | 3.9 (−4.6, 12.4) | 10.6 (0.5, 20.7) | 9.0 (−1.0, 19.0) |
| Visual reception | −3.5 (−11.1, 4.1) | −2.7 (−10.5, 5.1) | 4.3 (−4.7, 13.3) | 4.4 (−4.6, 13.3) |
| Fine motor | 2.8 (−4.8, 10.5) | 2.3 (−5.2, 9.9) | 3.8 (−5.3, 12.8) | 1.7 (−7.0, 10.4) |
| Receptive language | 0.7 (−4.5, 5.9) | −0.7 (−5.9, 4.5) | 3.5 (−2.8, 9.8) | 2.2 (−4.0, 8.5) |
| Expressive language | −0.4 (−6.3, 5.6) | −1.6 (−7.6, 4.4) | 4.7 (−2.4, 11.9) | 3.6 (−3.6, 10.8) |
| Composite | −0.3 (−9.5, 8.8) | −1.3 (−10.3, 7.7) | 8.2 (−2.6, 19.1) | 6.1 (−4.3, 16.5) |
AA, arachidonic acid; CI, confidence interval; DHA, docosahexaenoic acid. *Unadjusted models include a variable for laboratory. †Beta coefficient represents a change in fatty acid concentration of 1.0%. ‡Adjusted models include laboratory, infant sex, parity, smoking and preterm status.
In the models incorporating combinations of breast milk and formula DHA and AA concentrations, neither DHA, AA nor DHA : AA were associated with Mullen scores – all unadjusted and adjusted effect estimates were very close to 0 and none were statistically significant (α = 0.05) (e.g. DHA: adjusted β for composite = −0.5, 95% CI: −2.7, 1.7; AA: adjusted β for composite = 0.9, 95% CI: −1.5, 3.4) (Fig. 2, Table 4, results not shown for DHA : AA).
Figure 2.
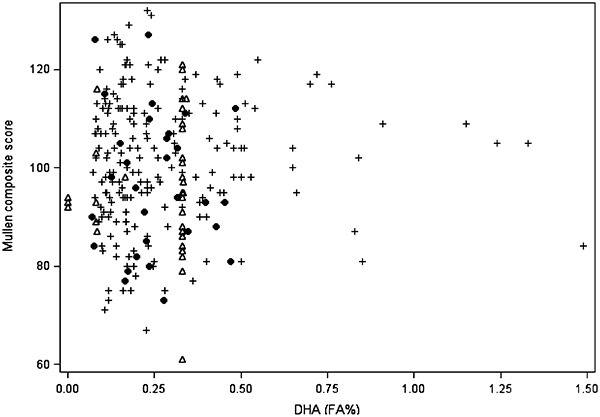
Calculated exposure to docosahexaenoic acid (DHA) from breast milk and infant formula averaged across 4 months plotted against Mullen Scales of Early Learning composite score. +, exclusively or almost exclusively breastfed; ●, partially breastfed; Δ, exclusively formula fed. PIN 2001–2006 [from PIN (1995–2006)] Babies Study.
Table 4.
Results from linear regression models for the association between docosahexaenoic acid and arachidonic acid in breast milk and infant formula weighted by feeding method for the first 4 post‐natal months and Mullen Scales of Early Learning scores, PIN 2001–2006 [from PIN (1995–2006)] Babies Study (n = 266)
| Mullen Scales of Early Learning | DHA in breast milk and formula (continuous) | AA in breast milk and formula (continuous) | ||
|---|---|---|---|---|
| β (CI) (unadjusted)*, † | β (CI) (adjusted) ‡ | β (CI) (unadjusted)*, † | β (CI) (adjusted) ‡ | |
| Gross motor | 1.3 (−0.7, 3.2) | 1.1 (−0.9, 3.1) | 1.4 (−0.9, 3.6) | 1.2 (−1.1, 3.4) |
| Visual reception | −0.2 (−2.0, 1.7) | −0.1 (−2.0, 1.8) | 0.8 (−1.3, 2.8) | 0.7 (−1.3, 2.8) |
| Fine motor | 0.5 (−1.4, 2.3) | 0.2 (−1.7, 2.0) | 0.3 (−1.7, 2.4) | 0.0 (−2.0, 2.0) |
| Receptive language | 0.3 (−1.0, 1.5) | −0.1 (−1.4, 1.2) | 0.4 (−1.0, 1.9) | 0.3 (−1.2, 1.7) |
| Expressive language | −0.2 (−1.6, 1.2) | −0.6 (−2.1, 0.8) | 1.0 (−0.6, 2.7) | 0.7 (−0.9, 2.3) |
| Composite | −0.0 (−2.3, 2.2) | −0.5 (−2.7, 1.7) | 1.2 (−1.3, 3.7) | 0.9 (−1.5, 3.4) |
AA, arachidonic acid; CI, confidence interval; DHA, docosahexaenoic acid. *Unadjusted models include a variable for laboratory. †Beta coefficient represents a change in fatty acid concentration of 1.0%. ‡Adjusted models include laboratory, feeding method for the first four post‐natal months, infant sex, parity, smoking, education, race/ethnicity and preterm status.
Discussion
Infants who were exclusively or almost exclusively breastfed for the first 4 months exhibited more advanced development at 12 months on several Mullen scales than exclusively formula‐fed infants, but estimates were attenuated after controlling for key confounders and were no longer statistically significant. This suggests that factors associated with breastfeeding, rather than the composition of breast milk, may contribute to positive associations previously reported. Other studies have reported similar null associations (Rogan & Gladen 1993; Innis et al. 1996), but some contrast our findings, noting enhanced development among breastfed infants (Morrow‐Tlucak et al. 1988; Temboury et al. 1994; Vestergaard et al. 1999; Agostoni et al. 2001; Pinelli et al. 2003; Caspi et al. 2007). Differences among studies may stem from differences in control for confounders and in the developmental tests used.
In our breast milk models, LCPUFA concentration was unrelated to development, given the small magnitude of the associations observed for DHA and the CI that included the null for the models for AA. The mean DHA and AA concentrations in our milk samples were similar to those from other US studies (Birch et al. 1998). A strength of this analysis is that by restricting to exclusively breastfed children, one avoids confounding from the choice of feeding method; this is one way this study contributes to the literature. Most studies of breast milk DHA have been maternal supplementation trials, and results have been equivocal (Gibson et al. 1997; Helland et al. 2001; Jensen et al. 2005; Lauritzen et al. 2005; Tofail et al. 2006; Judge et al. 2007). Studies could have null results because some developmental assessments may be insensitive to the effects of DHA, because intent‐to‐treat analyses were hindered by natural variability in DHA and AA within treatment groups from diet or other factors or because DHA and AA are simply not associated with development. Our observational study evaluated associations across the natural distribution of DHA and AA to ascertain whether differences might be detectable in these exposure ranges, as most US infants would have similar levels of exposure as those in this study. To our knowledge, this is the first study to take this approach. This study also contributes to the literature in its choice of developmental assessment tool – the Mullen distinguishes five developmental domains rather than assessing development globally.
Our models incorporating the DHA and AA concentration of breast milk with the DHA and AA concentration of formulas showed no association between the constructed exposure variables and development. We are aware of no previous studies that incorporated information about changing feeding methods across time with LCPUFA exposure from multiple sources. This exposure assessment method may better reflect the reality of feeding, where feeding methods and formula choices change over time.
Several weaknesses underlie our findings. First, very few infants were fed only formulas without DHA and AA for all 4 months to compare with infants fed only formulas with DHA and AA (although many infants were fed unsupplemented formulas for 1–3 months). Second, although our results reflect infants with a range of DHA exposure, reflecting some very low and very high concentrations (for example, 16 milk samples had <0.10% of total fatty acids as DHA and 26 had >0.50%), and this range is relatively wide compared with the exposures assigned by most formula trials, these measures may conceal associations at very high or very low exposure levels. Again, the participants in this observational study were free to choose any infant feeding method and maternal diet, so the fact that we observed mostly null results suggests that at the exposure levels in populations similar to this one, infants are unlikely to experience significant benefit from DHA. Third, the imprecise confidence limits reflect our limited participant sample size. However, even among more precise associations, most effect estimates were small or null. In addition, we observed differences in the distributions of LCPUFA concentrations between laboratories; these differences may affect interpretation of summary statistics (e.g. median) but should not affect the results of regression models. We adjusted for laboratory in all models to control for differences. Also, no participant characteristics varied by laboratory.
Our method of assessing exposure by combining breast milk and formula sources is novel but required some simplifying assumptions. We had to assume that breast milk composition was constant across lactation, and we could not quantify the amount of alpha‐linolenic acid in all formula products to estimate its contribution to the total available DHA. Also, we could not quantify the volume of milk or formula consumed; this is also a limitation of other studies that did not have information about volume. Around 4 months, some infants began receiving other foods which could be a source of LCPUFAs; however, typical baby foods were not a significant DHA source at the time of this study. Unless mixed with formula or breast milk, baby foods that replace milk or formula could reduce LCPUFA exposure. The accuracy of exposure measurement beyond 4 months of age may differ by the amount of solids fed, which could differ by initial feeding method, a problem for this study, as well as for trials that do not prescribe infant diet once solids are introduced. To minimize bias from differential exposure misclassification, we compared exposure for only the first 4 months. Assessing diet during this period precedes brain development at 12 months, which allows time biologically for DHA to influence the developing brain.
This study has strength in its ability to describe the association between real‐life feeding patterns and development. We evaluated infant development as it relates to the process of breastfeeding, the LCPUFA content of breast milk among exclusive breastfeeders, and the LCPUFA content of breast milk and formula combined, regardless of source. Most research in this area has been of randomized design supplementing either breastfeeding women or formulas. While proper randomization is an effective means of controlling confounding, if a non‐randomized breastfed group is compared with the randomized formula‐fed group(s), confounding by factors that underlie the choice about whether to breastfeed or to formula feed remains an issue. Randomized studies are especially good for investigating higher doses, but there is also selectivity among those who enter trials and are compliant with the protocols. This may affect the generalizability of the findings. We evaluated and controlled for many factors that underlie the choice of feeding method and did not select participants based on feeding method. Most randomized studies employ intent‐to‐treat analysis, a powerful technique, but intent‐to‐treat analysis assumes exposure is fixed within the treatment group and constant across the treatment period, which obscures the natural variation in most infants' diets. The observational design of this study enabled us to assess the impact of natural variability in DHA and AA in the population, stemming from variability in breast milk and formula concentrations, with extensive control for confounding.
Finally, it is possible that the Mullen may not detect subtle physiologic benefits from LCPUFAs. Most previous studies reporting positive associations between LCPUFAs and infant development have focused on global development, but some have focused on more specific aspects like achievement of gross motor milestones and language ability (Auestad et al. 2001; Agostoni et al. 2009). While the Mullen composite correlates strongly with the original Bayley Mental Development Index (correlations range 0.53–0.59) and moderately with the Psychomotor Development Index (correlations range 0.21–0.52), the Mullen subscales correspond to five specific developmental domains that are assessed together, allowing us to evaluate associations with particular domains that might be masked in a broader global measure that cannot detect uneven cognitive development (Mullen 1995). No previous studies in this area have used the Mullen.
Conclusion
After careful adjustment for confounding, neither the exclusivity of breastfeeding nor DHA and AA concentrations in breast milk and formula were associated with visual reception, language and motor skills, and overall cognitive development among infants at 12 months in this study. It remains possible that the effects of LCPUFAs are limited to certain outcomes like visual acuity or that lasting effects do not become apparent until after infancy. While supplementation trials are better suited to evaluating the effects of heavy LCPUFA supplementation or deficiency, this observational study design met our goal of evaluating natural variability in DHA and AA derived from population variability in milk concentration and formula use. Future studies of LCPUFA exposure and infant development should consider individual exposures using the actual concentrations of fatty acids in milk, as a complement to analyses that compare treatment groups.
Source of funding
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD37584, HD39373), the National Institute of Diabetes and Digestive and Kidney Diseases (DK61981, DK56350) and the National Institute of Environmental Health Sciences (P30ES10126) of the National Institutes of Health, the Carolina Population Center, and as part of the salary‐supported activities of extramural staff of the NICHD.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
All authors contributed to the conception, design, analysis and interpretation of the data and also the drafting of the paper. All authors critically reviewed the manuscript and have approved the final version.
Acknowledgements
We thank the women and children in the PIN studies, Kathryn Carrier for technical assistance, Kerry‐Ann da Costa for laboratory support and Diane Kaczor for programming support.
References
- Agostoni C., Marangoni F., Giovannini M., Galli C. & Riva E. (2001) Prolonged breast‐feeding (six months or more) and milk fat content at six months are associated with higher developmental scores at one year of age within a breast‐fed population. Advances in Experimental Medicine and Biology 501, 137–141. [DOI] [PubMed] [Google Scholar]
- Agostoni C., Zuccotti G.V., Radaelli G., Besana R., Podesta A., Sterpa A. et al (2009) Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: a randomized, prospective, double‐blind, placebo‐controlled trial. The American Journal of Clinical Nutrition 89, 64–70. [DOI] [PubMed] [Google Scholar]
- Auestad N., Halter R., Hall R.T., Blatter M., Bogle M.L., Burks W. et al (2001) Growth and development in term infants fed long‐chain polyunsaturated fatty acids: a double‐masked, randomized, parallel, prospective, multivariate study. Pediatrics 108, 372–381. [DOI] [PubMed] [Google Scholar]
- Birch E.E., Hoffman D.R., Uauy R., Birch D.G. & Prestidge C. (1998) Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research 44, 201–209. [DOI] [PubMed] [Google Scholar]
- Bligh E.G. & Dyer W.J. (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Carlson S.E., Werkman S.H. & Tolley E.A. (1996) Effect of long‐chain n‐3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. The American Journal of Clinical Nutrition 63, 687–697. [DOI] [PubMed] [Google Scholar]
- Caspi A., Williams B., Kim‐Cohen J., Craig I.W., Milne B.J., Poulton R. et al (2007) Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proceedings of the National Academy of Sciences of the United States of America 104, 18860–18865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2010) National immunization survey, provisional data, 2007 births. Available at: http://www.cdc.gov/breastfeeding/data/NIS_data/index.htm
- Clandinin M.T., Van Aerde J.E., Merkel K.L., Harris C.L., Springer M.A., Hansen J.W. et al (2005) Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 146, 461–468. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M. & Sagovsky R. (1987) Detection of postnatal depression. Development of the 10‐item Edinburgh postnatal depression scale. British Journal of Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Evenson K., Aytur S. & Borodulin K. (2009) Physical activity beliefs, barriers, and enablers among postpartum women. Journal of Women's Health 18, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell M.S., Morley R., Abbott R.A., Singhal A., Isaacs E.B., Stephenson T. et al (2002) Double‐blind, randomized trial of long‐chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 110, 73–82. [DOI] [PubMed] [Google Scholar]
- Fewtrell M.S., Abbott R.A., Kennedy K., Singhal A., Morley R., Caine E. et al (2004) Randomized, double‐blind trial of long‐chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. Journal of Pediatrics 144, 471–479. [DOI] [PubMed] [Google Scholar]
- Gibson R.A., Neumann M.A. & Makrides M. (1997) Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. European Journal of Clinical Nutrition 51, 578–584. [DOI] [PubMed] [Google Scholar]
- Helland I.B., Saugstad O.D., Smith L., Saarem K., Solvoll K., Ganes T. et al (2001) Similar effects on infants of n‐3 and n‐6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108, E82. [DOI] [PubMed] [Google Scholar]
- Innis S.M., Nelson C.M., Lwanga D., Rioux F.M. & Waslen P. (1996) Feeding formula without arachidonic acid and docosahexaenoic acid has no effect on preferential looking acuity or recognition memory in healthy full‐term infants at 9 mo of age. The American Journal of Clinical Nutrition 64, 40–46. [DOI] [PubMed] [Google Scholar]
- Jensen C.L., Voigt R.G., Prager T.C., Zou Y.L., Fraley J.K., Rozelle J.C. et al (2005) Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. The American Journal of Clinical Nutrition 82, 125–132. [DOI] [PubMed] [Google Scholar]
- Judge M.P., Harel O. & Lammi‐Keefe C.J. (2007) Maternal consumption of a docosahexaenoic acid‐containing functional food during pregnancy: benefit for infant performance on problem‐solving but not on recognition memory tasks at age 9 mo. The American Journal of Clinical Nutrition 85, 1572–1577. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Agranoff B.W., Radin N.S. & Burton R.M. (1969) Comparison of the fatty acids of lipids of subcellular brain fractions. Journal of Neurochemistry 16, 397–404. [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Jorgensen M.H., Olsen S.F., Straarup E.M. & Michaelsen K.F. (2005) Maternal fish oil supplementation in lactation: effect on developmental outcome in breast‐fed infants. Reproduction, Nutrition, Development 45, 535–547. [DOI] [PubMed] [Google Scholar]
- Makrides M., Neumann M.A., Simmer K. & Gibson R.A. (2000) A critical appraisal of the role of dietary long‐chain polyunsaturated fatty acids on neural indices of term infants: a randomized, controlled trial. Pediatrics 105, 32–38. [DOI] [PubMed] [Google Scholar]
- Makrides M., Gibson R.A., Mcphee A.J., Collins C.T., Davis P.G., Doyle L.W. et al (2009) Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid: a randomized controlled trial. Journal of the American Medical Association 301, 175–182. [DOI] [PubMed] [Google Scholar]
- Morrow‐Tlucak M., Haude R.H. & Ernhart C.B. (1988) Breastfeeding and cognitive development in the first 2 years of life. Social Science and Medicine 26, 635–639. [DOI] [PubMed] [Google Scholar]
- Mullen E. (1995) Mullen Scales of Early Learning. American Guidance Service: Circle Pines, MN. [Google Scholar]
- O'Connor D.L., Hall R., Adamkin D., Auestad N., Castillo M., Connor W.E. et al (2001) Growth and development in preterm infants fed long‐chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 108, 359–371. [DOI] [PubMed] [Google Scholar]
- PIN (19952006) Pregnancy, Infection, and Nutrition Study. PIN: Chapel Hill, NC. Available at: http://www.cpc.unc.edu/projects/pin [Google Scholar]
- Pinelli J., Saigal S. & Atkinson S.A. (2003) Effect of breastmilk consumption on neurodevelopmental outcomes at 6 and 12 months of age in VLBW infants. Advances in Neonatal Care 3, 76–87. [DOI] [PubMed] [Google Scholar]
- Rogan W.J. & Gladen B.C. (1993) Breast‐feeding and cognitive development. Early Human Development 31, 181–193. [DOI] [PubMed] [Google Scholar]
- Savitz D.A., Dole N., Williams J., Thorp J.M., Mcdonald T., Carter A.C. et al (1999) Determinants of participation in an epidemiological study of preterm delivery. Paediatrics & Perinatal Epidemiology 13, 114–125. [DOI] [PubMed] [Google Scholar]
- Scott D.T., Janowsky J.S., Carroll R.E., Taylor J.A., Auestad N. & Montalto M.B. (1998) Formula supplementation with long‐chain polyunsaturated fatty acids: are there developmental benefits? Pediatrics 102, E59. [DOI] [PubMed] [Google Scholar]
- Spielberger C. (1983) Manual for the State‐Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA. [Google Scholar]
- Sun G.Y. & Sun Y. (1972) Phospholipids and acyl groups of synaptosomal and myelin membranes isolated from the cerebral cortex of squirrel monkey (Saimiri sciureus). Biochimica et Biophysica Acta 280, 306–315. [DOI] [PubMed] [Google Scholar]
- Temboury M.C., Otero A., Polanco I. & Arribas E. (1994) Influence of breast‐feeding on the infant's intellectual development. Journal of Pediatric Gastroenterology and Nutrition 18, 32–36. [DOI] [PubMed] [Google Scholar]
- Tofail F., Kabir I., Hamadani J.D., Chowdhury F., Yesmin S., Mehreen F. et al (2006) Supplementation of fish‐oil and soy‐oil during pregnancy and psychomotor development of infants. Journal of Health, Population, and Nutrition 24, 48–56. [PubMed] [Google Scholar]
- US Department of Agriculture (2008) Nutrient Database for Standard Reference. U.S. Department of Agriculture: Washington, DC. [Google Scholar]
- Vestergaard M., Obel C., Henriksen T.B., Sorensen H.T., Skajaa E. & Ostergaard J. (1999) Duration of breastfeeding and developmental milestones during the latter half of infancy. Acta Paediatrica 88, 1327–1332. [DOI] [PubMed] [Google Scholar]


