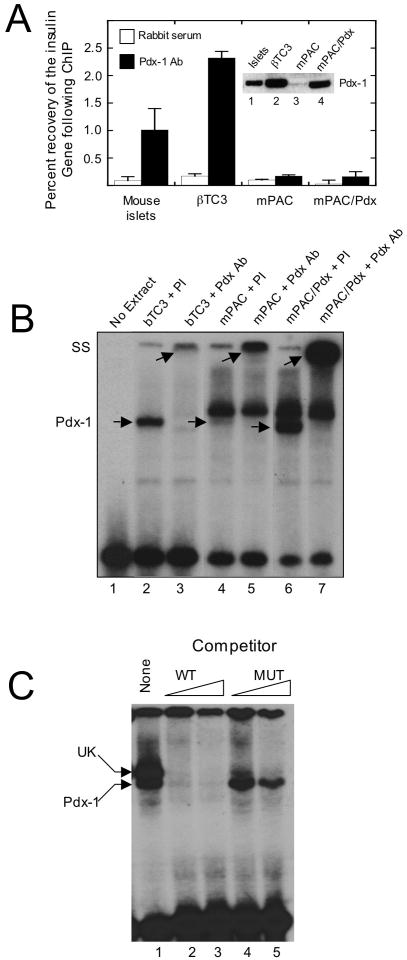
Figure 1. Association of Pdx-1 with the insulin promoter in vivo and in vitro.
A, extracts from mouse islets, βTC3 cells, mPAC cells, and mPAC cells overexpressing hamster Pdx-1 (mPAC/Pdx) were subject to ChIP using polyclonal Pdx-1 antiserum, and the recovery of the proximal insulin promoter (-126 to -296 bp relative to the transcriptional start site) was assessed by quantitative real-time PCR. Data are expressed as the percent of input DNA recovered following ChIP. Data represent the mean ± S.E. of at least three independent ChIP experiments; inset is an immunoblot showing Pdx-1 protein levels in islets, βTC3, mPAC, and mPAC/Pdx cells. B, representative EMSA using nuclear extracts isolated from βTC3, mPAC, and mPAC/Pdx cells and a 32P-labeled oligonucleotide probe containing the mouse insulin 1 A4/A3 element; lower arrows indicate positions of the Pdx-1 complex and the upper arrows indicate positions of the supershifted complex upon addition of Pdx-1 antiserum; PI, preimmune serum; Pdx Ab, polyclonal Pdx-1 antiserum; SS, supershift. C, representative EMSA using nuclear extract from mPAC/Pdx cells, a 32P-labeled oligonucleotide containing the insulin 1 A4/A3 element, and increasing concentrations (100- or 1000-fold excess) of unlabeled oligonucleotide (WT) or unlabeled oligonucleotide containing a mutation in the A4/A3 element (MUT), as indicated in Materials and Methods; upper arrow indicates positions of the unknown (UK) protein complex, and lower arrow indicates position of the Pdx-1 complex.