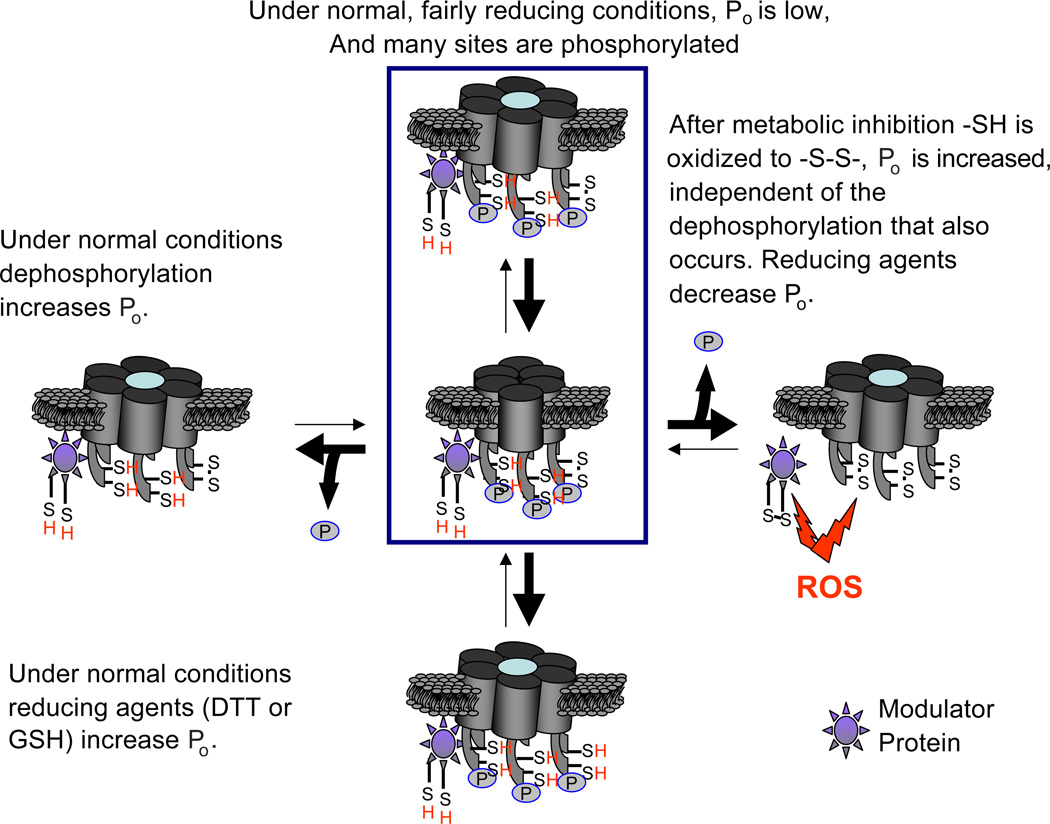
Fig. 1.
Diagram of hemichannels depicting two putative covalent modifications, disulfide bond formation and phosphorylation, that the regulate open probability ( Po) of Cx43 hemichannels. Under normal conditions, most cysteines are in their reduced form, most potential sites are phosphorylated, and hemichannel Po is low. Dephosphorylation under normal conditions increases Po (left hemichannel). Metabolic inhibition generates reactive oxygen species, e.g., nitric oxide, that oxidize cystines to cysteines and increase Po. Dephosphorylation induced by metabolic inhibition appears not to be necessary for this opening (right hemichannel). Under normal conditions, reducing agents, DTT and GSH, increase Po, perhaps by reducing disulfide bonds formed under basal conditions (lowest hemichannel). Phosphorylation state may account for different effects of reducing agents in normal conditions and under metabolic inhibition. Reducing agents may produce different covalent changes depending on condition. Modulator proteins may participate in these actions. P: phosphate group on Cx43. –S–S–: disulfide bond. ROS: reactive oxygen species.