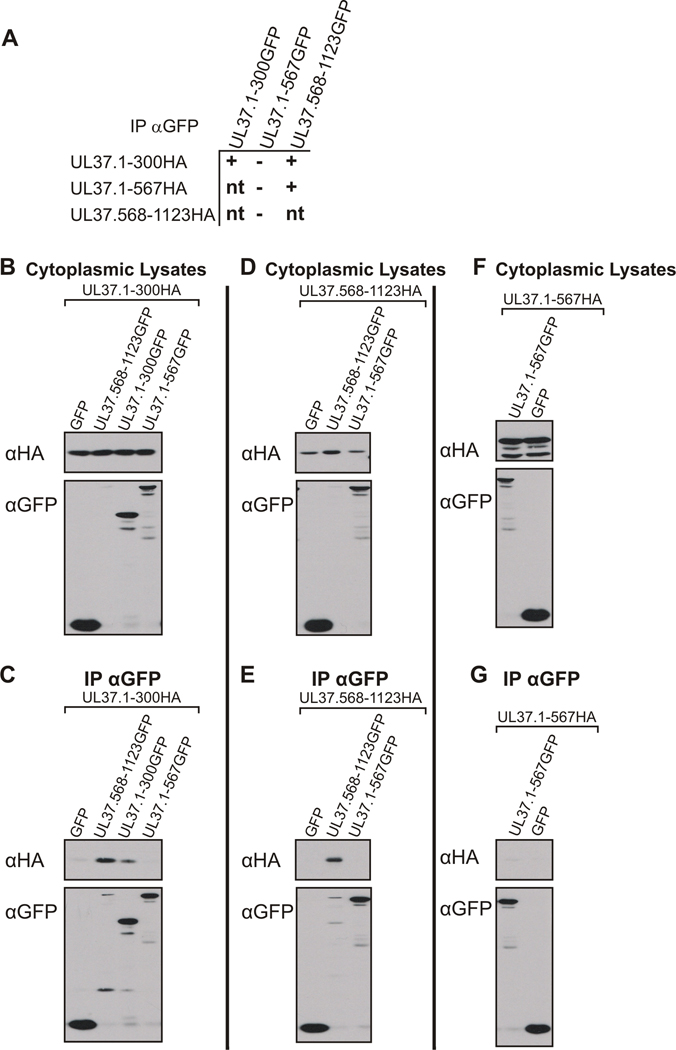
Figure 6. Interactions of Amino- and Carboxy-Terminal UL37 Self-Association Domains Analyzed by Coimmunoprecipitation-Western Blot Analysis.
(A) A schematic representation of the UL37 N-terminal and C-terminal HA and GFP fusion constructs used in the coimmunoprecipitation assays. A tabular summary of results indicate the UL37GFP truncation mutants that bind (+) or do not bind (−) UL37HA truncation mutants in coimmunoprecipitation assays. Possible combinations of coimmunoprecipitations that were not tested are indicated by “nt.” (B, D, F) Expression of cotransfected UL37HA and UL37 GFP mutants. Vero cells were cotransfected with the indicated plasmids, harvested at approximately 24 h post-transfection and lysed in NP40 lysis buffer. A portion of each cytoplasmic lysate was analyzed for protein expression using rabbit antibodies to HA (αHA, top panels) and GFP (αGFP, bottom panels). (C, E, G) Coimmunoprecipitation of UL37 HA-tagged and UL37 GFP-tagged mutant proteins. Coimmunoprecipitations were performed and analyzed as described in the legend to Fig. 5. Western blotting with rabbit anti-HA (αHA, top panels) and anti-GFP (αGFP, bottom panels) antibodies was used to determine if the UL37HA mutants coimmunoprecipitated with the GFP fusion constructs.