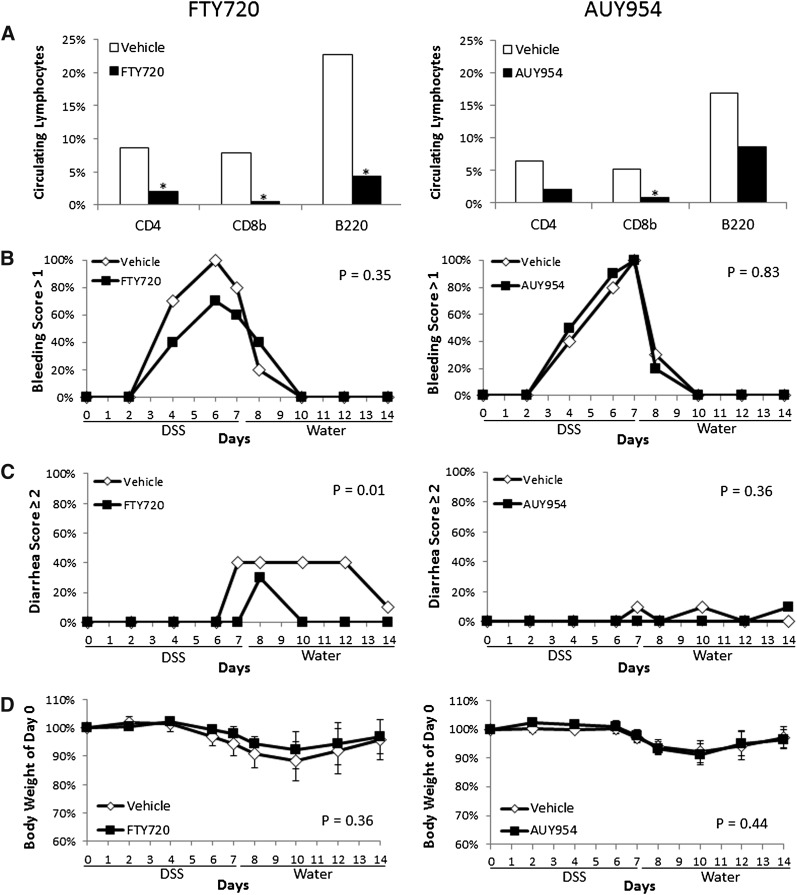
Fig. 5.
FTY720 and AUY954 administration does not enhance DSS-induced bleeding. A: The percent of circulating lymphocytes was examined after 14 days of daily administration of FTY720 (3 mg/kg) or AUY954 (1 mg/kg) relative to vehicle-treated mice (n = 4–5/group), under control conditions. A statistically significant reduction in the percent of CD4-, CD8b-, and B220-expressing cells [median (range)] was found in FTY720-treated mice [CD4: 2.0 (0.7–5.0)], [CD8b: 0.5 (0.3–0.7)], [B220: 4.3 (3.7–11.9)] compared with controls [CD4: 8.6 (6.5–10.4)], [CD8b: 7.9 (6.0–9.3)], [B220: 22.7 (16.6–26.6)]. A statistically significant reduction in the percent of CD8b-expressing cells [median (range)] was found in AUY954-treated mice [0.8 (0.6–1.4)] compared with controls [5.2 (4.8–5.50] with a trend for a reduction in CD4 and B220-expressing cells [CD4: 2.1 (1.4–8.7)], B220: [8.6 (3.4–18.3)] vs. [CD4: 6.4 (5.5–7.2)], B220: [16.9 (13.9–21.8)]. (B–D) To assess whether either drug had effects on the severity of experimental colitis, 2% DSS was administered to wild-type mice for 7 days, then mice were switched to plain drinking water for an additional 7 days. FTY720, AUY954, or their respective vehicles were administered daily during the entire 14 day experimental period (n = 10/group). Clinical endpoints of colitis severity including bleeding (B), diarrhea (C) and body weight change (D) were measured during disease progression. Error bars represent SD. Differences in bleeding or diarrhea severity were determined by quantifying the percent of mice with a score >1 or ≥2, respectively, in each group. Differences in body weight were determined by quantifying the maximum weight loss of individual mice in each group.