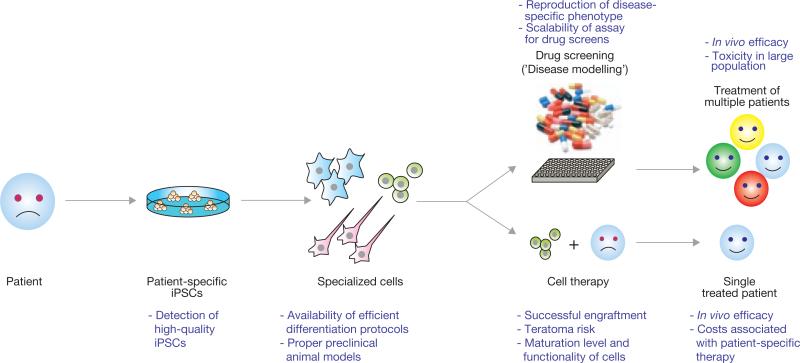
Figure 1.
Schematic representation of the potential utility of iPSC technology in regenerative medicine. Introduction of reprogramming factors, such as Oct4, Sox2, Klf4 and c-Myc, into somatic cells of patients (for example, skin cells, keratinocytes or blood cells) gives rise to iPSCs. These patient-specific iPSCs can then be differentiated into a variety of specialized cell types for a potential use in disease modelling (top) or cell therapy (bottom). The concept behind disease modelling is to reproduce a cellular phenotype in cultured iPSC-derived cells as it occurs in the patient. Such a phenotype could be employed to model this disease for mechanistic studies as well as for large-scale drug screening efforts to identify compounds that could be used to treat any patient suffering from the same disease. The idea behind cell therapy is to generate autologous specialized cells from iPSCs for transplantation into individual patients. Shown in purple are the current limitations in using iPSC technology in regenerative medicine.