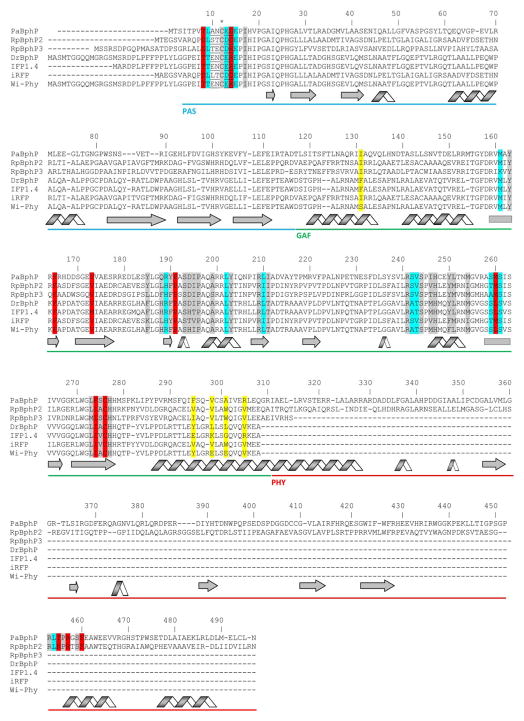
Fig. 3.
Alignment of amino acid sequences of the photosensory modules of the most characterized BphPs. The proteins were chosen based on the availability of the crystal structures (PaBphP, RpBphP3, DrBphP) and those that were developed to the fluorescent proteins (IFP1.4, iRFP, Wi-Phy, and RpBphP2 as the template for iRFP). The numbering of amino acid residues follows that for the PaBphP protein. Cys residue, which is covalently attached to the BV chromophore, is marked with asterisk. The chromophore surrounding residues within 4.5 Å, 4.5–5.5 Å and 5.5–6.5 Å are highlighted with gray, cyan, and red colors, respectively. The residues located in the dimer interface are highlighted with yellow. The residues located in the close proximity to the thioether bond between BV and apoprotein are underlined. The α-helixes and β-sheets demonstrate the secondary structure of BphPs. The PAS, GAF and PHY domains are underlined with the blue, green, and red lines, respectively.