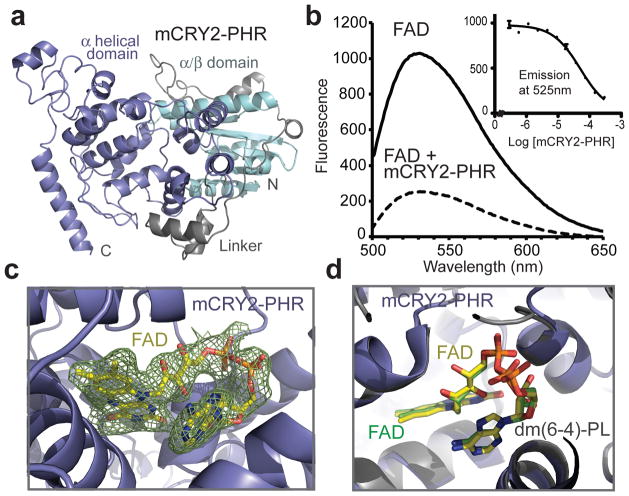
Figure 1. Structure of mCRY2-PHR in apo- and FAD-bound forms.
a, Overall structure of the apo mCRY2-PHR domain. b, mCRY2-PHR-induced quenching of FAD fluorescence. Inset shows FAD fluorescence quenching with mCRY2-PHR concentration titrated (error bars representing s.d.). c, A close-up view of FAD bound to mCRY2-PHR with positive Fo − Fc electron density contoured at 1.5 σ and calculated before the cofactor was built (green mesh). d, mCRY2-PHR and Drosophila (6-4)-photolyase are superimposed with their FAD cofactors shown in sticks and their FAD carbon atoms colored in yellow and green, respectively.