Abstract
Aspergillus colonization after lung transplantation may increase the risk for bronchiolitis obliterans syndrome (BOS), a disease of small airways. We hypothesized that colonization with small conidia Aspergillus species would be associated with a greater risk of BOS, based upon an increased likelihood of deposition in small airways. We studied adult primary lung recipients from two large centers; 298 recipients at University of California, Los Angeles and 482 recipients at Duke University Medical Center. We grouped Aspergillus species by conidia diameter ≤3.5μm. We assessed the relationship of colonization with outcomes in Cox models. Pre-BOS colonization with small conidia Aspergillus species, but not large, was a risk factor for BOS (P = 0.002, HR 1.44, 95% CI 1.14–1.82), along with acute rejection, single lung, and Pseudomonas. Colonization with small conidia species also associated with risk of death (P = 0.03, HR 1.30, 95% CI 1.03–1.64). Although other virulence traits besides conidia size may be important, we have demonstrated in two large independent cohorts that colonization with small conidia Aspergillus species increases the risk of BOS and death. Prospective evaluation of strategies to prevent Aspergillus colonization of small airways is warranted, with the goal of preserving lung allograft function as long as possible.
Keywords: lung transplantation, BOS, bronchiolitis obliterans syndrome, Aspergillus
Long-term survival after lung transplantation is largely dependent on the avoidance of bronchiolitis obliterans syndrome (BOS), the predominant cause of chronic lung allograft dysfunction (1, 2). BOS correlates histologically with obliterative bronchiolitis (OB), which describes fibrosis and luminal occlusion of membranous and respiratory bronchioles (i.e. small airways) (3, 4). Clinically, BOS manifests as progressive and irreversible airflow obstruction (5, 6). Within 5 years of transplant, nearly half of all lung transplant recipients develop BOS (7). Once BOS ensues, quality of life is diminished and the median survival is approximately 2.5 years (8). While acute rejection (AR) is considered the principal risk factor, there is growing evidence that pulmonary infections, especially with cytomegalovirus (CMV), Pseudomonas, and community acquired respiratory viruses (CARV), increase the risk for developing BOS (9–13). Recently, pulmonary colonization with Aspergillus species has also been implicated as a potential risk factor for the development of BOS (14).
In the environment outside of a host, Aspergillus produces conidia (asexual spores) that can be easily dispersed in the air. Inhalation of aerosolized conidia is usually the initial route of entry for Aspergillus infection. The size of conidia varies from species to species, and this trait is believed to affect the likelihood of inhaled conidia depositing in the proximal airways versus the lung periphery (small airways and alveoli) (15). Indeed, particle size is known to affect the site of airways deposition with particles in the 1–3 μm range being best suited to reach and be deposited in the lung periphery while larger particles are increasingly likely to be deposited in the more proximal airways (16–19). Given that the pathologic lesion responsible for BOS occurs in the small airway, we hypothesized that small conidia Aspergillus species would be associated with a greater risk for BOS based upon a greater likelihood that the small conidia would be deposited in and colonize the small airways.
We sought to determine and validate the significance of colonization with small and large conidia Aspergillus species after lung transplantation. While previous studies have reported relationships between respiratory infections and BOS, all have been single center studies often with relatively small numbers of patients included. Here we report findings from two large independent cohorts of lung transplant recipients, validating a relationship between small conidia Aspergillus species colonization and BOS. The institutional review boards at both the University of California Los Angeles (UCLA) and Duke University Medical Center (DUMC) approved this study. Some of the results have previously been reported in the form of an abstract (20).
METHODS
Recipient Cohorts
UCLA
We reviewed all adult lung transplant recipients transplanted at UCLA between 1/1/2000 and 6/30/2009. For inclusion in this study, lung recipients had to have at least 1 posttransplant bronchoscopy and 6 posttransplant pulmonary function test (PFT) measurements. Retransplant and multiorgan recipients were excluded. During this period, 368 adult lung transplant operations were performed at UCLA and 298 recipients were included in the final cohort. Reasons for exclusion are shown in Figure 1. Outcomes were collected through December 31, 2010, providing a minimum follow up of 1.5 years.
Figure 1.
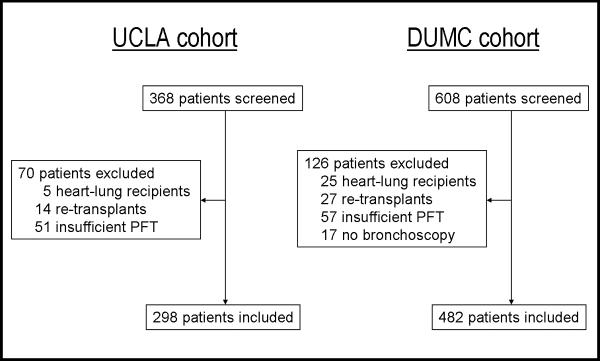
Derivation of the study cohorts and reasons for exclusion. PFT = pulmonary function testing.
DUMC
Identical inclusion and exclusion criteria were applied to all adult lung recipients transplanted at DUMC between 1/1/1998 and 10/1/2008. During this period, 608 adult lung transplant operations were performed and 482 recipients were included in the final cohort (Figure 1). Outcomes were collected through October 1, 2010, providing a minimum follow up of two years.
Clinical Management
The management of recipients at both centers have been described elsewhere and are briefly summarized below (14), (21).
At UCLA, induction immune suppression included rabbit Anti-Thymocyte-Globulin (ATG) or a CD25 antagonist (≥65 years old, prior malignancy, or chronic infection). Thereafter, patients were maintained on triple immunosuppression with tacrolimus, mycophenolate, and corticosteroids. Surveillance bronchoscopies and transbronchial biopsies (TBBXs) were performed at 1, 3, 6, and 12 months, and as clinically indicated. PFTs were performed at each clinic visit, usually every 1–2 weeks during the first 3 months after transplantation and at least quarterly thereafter. Antifungal prophylaxis included nebulized amphotericin B 15 mg BID (prior to 10/03) or nebulized amphotericin B lipid complex 50 mg/day for 3 days and then weekly (10/03 to present) plus IV caspofungin 50 mg/day (9/02 to present) for the duration of the post-operative hospitalization. Patients who were colonized prior to transplant received azole antifungal therapy (itraconazole, voriconazole, posaconazole). Posttransplant positive fungal cultures were treated similarly with azole antifungal therapy.
At DUMC, induction and maintenance immunosuppression consisted of ATG (prior to January 1999) or a CD25 antagonist (after 1999), followed by triple immunosuppression with cyclosporine (prior to 2002) or tacrolimus (after 2002), azathioprine, and corticosteroids, respectively. Bronchoscopies and TBBXs were performed at standardized intervals: 1, 3, 6, 9, and 12 months, and annually thereafter or as clinically indicated. PFTs were administered weekly in the first six weeks of transplant and quarterly thereafter, concomitant with clinic visits. Antifungal prophylaxis included nebulized amphotericin B 15 mg BID (prior to 1/01) or nebulized amphotericin B lipid complex 50 mg administered daily for 4 days then weekly for the duration of the post-operative hospital course. Patients who were colonized prior to transplant received azole antifungal therapy. Posttransplant positive fungal cultures were treated similarly with azole antifungal therapy.
Both institutions treat acute rejection episodes with pulse dose methylprednisolone (1 gram/day) for 3 days followed by increased oral prednisone (0.5–1 mg/kg/day) tapered over 6–8 weeks.
Definitions of Aspergillus Colonization
Aspergillus colonization was defined by a single positive posttransplant respiratory culture (bronchoalveolar lavage, sputum, or endotracheal tube suction), consistent with published consensus definition for fungal infections (22). Aspergillus species were grouped as either small or large conidia species according to published conidia diameter (23). Species with an average conidia diameter ≤3.5 μm were specified as small conidia species (A. fumigatus [2.5–3 μm], A nidulans [3–3.5 μm], A. terreus [1.8–2.4 μm], and A. flavipes [2–3 μm]) and those with an average conidia diameter >3.5 μm were grouped as large conidia species (A. niger [4–5 μm], A. flavus [3.5–4.5 μm], A. ustus [3.2–4.5 μm], and A. clavatus [3–4.5 μm]).
Definitions of CMV Pneumonitis, Pseudomonas, CARV, AR and BOS
CMV pneumonitis was defined by the presence of one or more positive CMV infected cells on immunoperoxidase staining. Every TBBX was prospectively assessed for invasive CMV pneumonitis. Pseudomonas infection was defined by a single positive posttransplant respiratory culture for Pseudomonas aeruginosa. CARV infection (RSV, parainfluenza (1, 2, and 3), influenza (A and B), or adenovirus) was diagnosed using direct fluorescent antigen testing, shell vial, and standard cell culture methods. Polymerase chain reaction (PCR) assays for respiratory viruses were not in use in either center during the period of this study.
An experienced lung transplant pathologist from each center prospectively evaluated and graded each TBBX for AR (A grades 0–4) according to International Society for Heart and Lung Transplantation (ISHLT) guidelines (3, 4). BOS was defined in accordance with ISHLT diagnostic criteria (24). BOS grade ≥1 assignment was limited to patients with at least 6 PFTs to ensure that a sufficient number of FEV1 measurements were used to estimate the posttransplant baseline and to observe a sustained decline. BOS free days were defined as time from transplant to the onset of BOS or, in the case of BOS free patients, time from transplant to the last PFT date on record.
Statistical Analysis
Recipient characteristics were described as median and interquartile range when appropriate and analyzed using nonparametric Wilcoxon tests. Categorical variables were analyzed using chi-square tests. Kaplan-Meier methods were used to describe estimates of the incidence of events over time.
Standard Cox proportional hazards models were used to identify risk factors for pre-BOS colonization with small or large conidia Aspergillus species. Colonization with small or large conidia Aspergillus species were considered in separate models. Aspergillus events occurring after BOS were ignored and Aspergillus free days were censored at the time of BOS onset or at the last follow-up on record (for BOS free patients). Baseline characteristics were tested in univariable models, and potential predictors (P ≤ 0.10) were then included in multivariable models. These analyses used joined data from both centers and the multivariable models were adjusted for center.
Cox models were also used to assess the relationship between Aspergillus colonization and other clinical variables with BOS and mortality. In these models, colonization with either small or large conidia Aspergillus species were modeled as two separate time-dependent variables, modeling the change in status once a colonization event occurred. CMV pneumonitis, Pseudomonas, and CARV were also modeled as time-dependent variables in this manner. BOS was modeled as a time-dependent variable in the Cox models of mortality. AR was modeled as the AR ratio to account for severity and sampling variation in patients over time as previously described (9). Briefly, the AR ratio was determined by summing all A grades occurring prior to the outcome of interest (BOS or death) or the last follow-up date in cases where the outcome had not occurred. This sum was then divided by the number of evaluable TBBXs, summed up to the outcome of interest or last follow-up. Analyses were performed stratified by center and in the joined cohort. Colonization variables for small and large conidia species, plus all potential predictors (P ≤ 0.10) from any univariable analysis was then included in the multivariable models.
Statistical significance was defined as a two-tailed P value less than or equal to 0.05. Proportional hazard assumptions were assessed and satisfied for all Cox proportional hazards models. All analyses were conducted with SAS statistical software version 9.1.3 (Cary, NC).
RESULTS
Study Patient Characteristics
The demographic and clinical characteristics of the recipient cohorts are shown in Table 1. The UCLA and DUMC cohorts differed in many regards. UCLA transplanted a greater proportion of older patients and those with restrictive lung disease, while obstructive lung disease and cystic fibrosis/bronchiectasis were more common indications for transplant at DUMC. UCLA performed single lung transplant more frequently than DUMC. UCLA used ATG for induction in patients under age 65 and basiliximab in patients 65 and older. In contrast, DUMC discontinued the use of ATG as an induction agent in 1999 and has since used basiliximab in all patients. Pre-BOS CMV pneumonitis occurred less frequently at UCLA than DUMC, while Pseudomonas and CARV infections occurred with similar frequency at each center. AR was more frequently diagnosed at DUMC. However, among those with at least one episode of AR, the burden of AR, represented by the AR ratio, was higher in the UCLA cohort. Kaplan Meier one, three, and five year BOS estimates for UCLA and DUMC were 8%, 41%, and 58%, and 6%, 23%, and 45%, respectively. Kaplan Meier one, three, and five year survival estimates for UCLA and DUMC were 95%, 72%, and 54%, and 97%, 83%, and 63%, respectively. Patients were followed a median of 3.3 years (IQR = 2.0 – 4.9) at UCLA and 4.3 years (IQR = 2.7 – 6.5) at DUMC.
TABLE 1.
CLINICAL CHARACTERISTICS OF THE STUDY COHORTS
| UCLA Cohort (n = 298) | DUMC Cohort (n = 482) | P value | |
|---|---|---|---|
| Female Gender | 41% (123) | 43% (209) | 0.57 |
| Age at transplant, IQR | 60 (55–65) | 56 (42–62) | <0.001 |
| Age ≥ 65 years | 27% (81) | 12% (60) | <0.001 |
| Race | |||
| Caucasian | 78% (232) | 89% (429) | <0.001 |
| African American | 6% (18) | 9% (45) | 0.10 |
| Hispanic American | 10% (29) | 1% (4) | <0.001 |
| Other | 6% (19) | 1% (4) | <0.001 |
| Native Disease | |||
| Restrictive | 56% (166) | 33% (159) | <0.001 |
| Obstructive | 35% (105) | 45% (219) | 0.005 |
| Cystic/bronchiectasis | 6% (17) | 18% (89) | <0.001 |
| Vascular | 3% (10) | 4% (15) | 0.85 |
| Bilateral Transplant | 59% (175) | 90% (434) | <0.001 |
| Induction Agent | |||
| Antithymocyte globulin | 58% (174) | 6% (29) | <0.001 |
| Basiliximab | 42% (124) | 94% (453) | <0.001 |
| CMV Mismatch (D+/R−) | 22% (65) | 21% (103) | 0.88 |
| Pre-BOS CMV pneumonitis 1yr, 3yr*€ | 1%, 2% | 25%, 29% | <0.001 |
| Pre-BOS Pseudomonas 1yr, 3yr*€ | 22%, 28% | 22%, 25% | 0.24 |
| Pre-BOS CARV 1yr, 3yr*€ | 5%, 9% | 7%, 11% | 0.32 |
| Pre-BOS AR 1yr, 3yr*€ | 53%, 63% | 70%, 80% | <0.001 |
| Median Pre-BOS AR Ratio, IQR*‡ | 0.50 (0.33–0.74) | 0.40 (0.25–0.60) | <0.001 |
Event occurring prior to BOS, or for BOS-free patient, occurring at any point over follow-up.
Where perivascular grade ≥ A1
Among patients with at least 1 Pre-BOS AR episode
Kaplan-Meier estimates of incidence.
Abbreviations: IQR: interquartile range, CMV: cytomegalovirus, BOS: bronchiolitis obliterans syndrome, AR: acute rejection.
Incidence and Predictors of Aspergillus Colonization
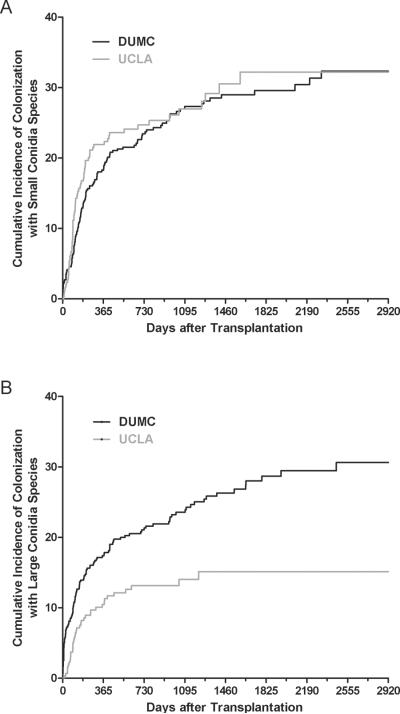
In both centers, prior to and after the diagnosis of BOS, Aspergillus fumigatus was the most common small conidia species and Aspergillus niger was the most common large conidia species (Table 2). The incidence over time of pre-BOS colonization with small conidia Aspergillus species was similar at each center (P = 0.49) (Figure 2). Kaplan-Meier estimates of pre-BOS colonization with small conidia Aspergillus species at 1-year were 22% and 19% at UCLA and DUMC, respectively. There was a lower incidence over time of pre-BOS colonization with large conidia Aspergillus species at UCLA compared with DUMC (P = 0.0007) (Figure 2). One-year Kaplan-Meier estimates of colonization with large conidia Aspergillus species were 10% and 17% at UCLA and DUMC, respectively. Among those colonized at UCLA, the median time to pre-BOS colonization with small species was 113 days (IQR 74–218) and with large species was 118 days (IQR 72–297). Among those colonized at DUMC, the median time to pre-BOS colonization with small species was 185 days (IQR 101–426) and with large species was 125 days (IQR 23–424).
TABLE 2.
FIRST COLONIZING ASPERGILLUS SPECIES ACCORDING TO CENTER AND CONIDIA SIZE
| UCLA Cohort |
DUMC Cohort |
Joined Cohort |
||||
|---|---|---|---|---|---|---|
| Pre-BOS | All | Pre-BOS | All | Pre-BOS | All | |
| Small conidia species | 76 | 94 | 130 | 149 | 206 | 243 |
| A. fumigatus | 63 (83%) | 76 (81%) | 114 (88%) | 133 (89%) | 177 (86%) | 209 (86%) |
| A. terreus | 4 (5%) | 5 (5%) | 9 (7%) | 9 (6%) | 13 (6%) | 14 (6%) |
| A. nidulans | 5 (7%) | 7 (8%) | 3 (2%) | 3 (2%) | 8 (4%) | 10 (4%) |
| Other species | 4 (5%) | 5 (5%) | 2 (1.5%) | 2 (1.5%) | 6 (3%) | 7 (3%) |
| A. fumigatus + other sp. | 0 | 1 (1%) | 2 (1.5%) | 2 (1.5%) | 2 (1%) | 3 (1%) |
| Large conidia species | 38 | 47 | 105 | 120 | 143 | 167 |
| A. niger | 25 (66%) | 32 (68%) | 74 (70%) | 84 (70%) | 99 (69%) | 116 (70%) |
| A. flavus | 9 (24%) | 9 (19%) | 26 (25%) | 31 (26%) | 35 (25%) | 40 (24%) |
| A. clavatus | 0 | 0 | 4 (4%) | 4 (3%) | 4 (3%) | 4 (2%) |
| A. ustus | 2 (5%) | 4 (9%) | 1 (1%) | 1 (1%) | 3 (2%) | 5 (3%) |
| A. niger + A. flavus | 2 (5%) | 2 (4%) | 0 | 0 | 2 (1%) | 2 (1%) |
Figure 2.
Kaplan-Meier estimates of cumulative incidence of Aspergillus colonization with small and large conidia species by center. (A) The incidence of colonization with small conidia Aspergillus species over time was similar at both UCLA and DUMC (P = 0.49). (B) The incidence of colonization with large conidia Aspergillus species over time was greater at DUMC as compared to UCLA (P = 0.007).
We explored potential risk factors for pre-BOS Aspergillus colonization with small and large conidia species in the joined cohort (UCLA and DUMC combined). Among all baseline characteristics, only advanced age (≥65 years) at the time of transplantation was a significant risk factor for colonization with small conidia Aspergillus species in the univariable joined cohort analysis (P = 0.004, HR 1.64, 95% CI 1.17–2.25) (Table 3). There was also a nonsignificant trend seen with prophylaxis era: there was a trend for increased risk of colonization with small conidia species after the protocol shift to nebulized Amphotericin B lipid complex (P = 0.10, HR 1.34, 95% CI 0.95–1.96). Advanced age remained a significant predictor of pre-BOS colonization with small conidia Aspergillus species in the multivariable model including adjustment for center (P = 0.01, HR 1.58, 95% CI 1.12–2.19). Bilateral lung transplant exclusively was associated with an increased risk for colonization with large conidia Aspergillus species in univariate joined cohort analysis (P = 0.001, HR 2.16, 95% CI 1.36–3.65) (Table 3). After adjustment for center, bilateral lung transplant remained a significant risk factor for colonization with large conidia Aspergillus species (P = 0.02; adjusted HR 1.79, 95% CI 1.10–3.08).
TABLE 3.
ASSOCIATION OF BASELINE CHARACTERISTICS WITH PRE-BOS ASPERGILLUS COLONIZATION ACCORDING TO CONIDIA SIZE – JOINED COHORT
| Small Conidia Asp Species |
Large Conidia Asp Species |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Univariable models | ||||||
| Female Gender | 1.09 | 0.83–1.43 | 0.55 | 1.04 | 0.76–1.42 | 0.81 |
| Age ≥ 65 | 1.64 | 1.17–2.25 | 0.004 | 0.98 | 0.63–1.47 | 0.94 |
| Race | ||||||
| Caucasian | 1.30 | 0.83–2.16 | 0.26 | 0.72 | 0.48–1.16 | 0.17 |
| African American | 0.66 | 0.36–1.11 | 0.12 | 1.36 | 0.81–2.17 | 0.24 |
| Native Disease | ||||||
| Restrictive | 1.02 | 0.77–1.34 | 0.91 | 0.98 | 0.71–1.35 | 0.92 |
| Obstructive | 1.01 | 0.76–1.33 | 0.96 | 1.07 | 0.78–1.47 | 0.67 |
| CF/bronchiectasis | 0.92 | 0.61–1.35 | 0.69 | 0.92 | 0.57–1.42 | 0.73 |
| Vascular | 1.23 | 0.56–2.33 | 0.58 | 0.99 | 0.35–2.17 | 0.98 |
| Bilateral Transplant | 0.87 | 0.63–1.21 | 0.39 | 2.16 | 1.36–3.65 | 0.001 |
| ATG Induction | 0.89 | 0.67–1.16 | 0.39 | 0.96 | 0.70–1.31 | 0.80 |
| CMV Mismatch (D+/R−) | 0.94 | 0.66–1.30 | 0.69 | 0.89 | 0.59–1.30 | 0.57 |
| Center = DUMC | 0.91 | 0.68–1.21 | 0.50 | 1.86 | 1.30–2.72 | 0.001 |
| Prophylaxis Era (ABLC) | 1.34 | 0.95–1.96 | 0.10 | 1.21 | 0.83–1.86 | 0.33 |
| Mulivariable models | ||||||
| Age ≥ 65 | 1.58 | 1.12–2.19 | 0.01 | - | - | - |
| Prophylaxis Era (ABLC) | 1.25 | 0.88–1.83 | 0.22 | - | - | - |
| Center = DUMC | 0.98 | 0.73–1.31 | 0.85 | 1.61 | 1.11–2.38 | 0.01 |
| Bilateral Transplant | - | - | - | 1.79 | 1.10–3.08 | 0.02 |
Abbreviations: Asp: Aspergillus CF: cystic fibrosis. ATG: anti-thymocyte globulin. CMV: cytomegalovirus, DUMC: Duke University Medical Center.
Colonization with Small Conidia Aspergillus Species is Associated with BOS
In univariable Cox models for BOS, colonization with small conidia Aspergillus species was significantly associated with an increased risk of BOS in each center, and in the joined cohort (P < 0.001, unadjusted HR 1.53, 95% CI 1.22–1.93) (Table 4). Colonization with large conidia Aspergillus species exhibited no relationship with BOS in either center or the joined cohort. Other potential risk factors for BOS (P ≤ 0.10) in at least one center or the joined cohort included age ≥65 years, single lung transplant, AR ratio, CMV pneumonitis, Pseudomonas infection, and CF/bronchiectasis (negative association). Each of these variables was included in the final multivariable models.
TABLE 4.
COX MODELS FOR RISK OF BRONCHIOLITIS OBLITERANS SYNDROME
| UCLA Cohort |
DUMC Cohort |
Joined Cohort* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Univariable Models | ||||||
| Female Gender | 1.00 (0.71–1.42) | 0.99 | 1.12 (0.86–1.46) | 0.40 | 1.07 (0.87–1.32) | 0.53 |
| Age ≥ 65 | 1.15 (0.77–1.72) | 0.49 | 1.47 (0.99–2.18) | 0.05 | 1.45 (1.10–1.91) | 0.009 |
| Diagnosis | ||||||
| Restrictive/other | Reference | Reference | Reference | |||
| Obstructive | 1.31 (0.91–1.88) | 0.15 | 0.89 (0.67–1.20) | 0.44 | 0.97 (0.77–1.22) | 0.78 |
| CF/bronchiectasis | 0.69 (0.32–1.50) | 0.35 | 0.74 (0.50–1.09) | 0.13 | 0.69 (0.49–0.97) | 0.03 |
| Single Lung Txp | 1.32 (0.93–1.88) | 0.12 | 1.83 (1.28–2.61) | <0.001 | 1.78 (1.34–2.26) | <0.001 |
| ATG Induction | 1.06 (0.75–1.53) | 0.73 | 1.12 (0.86–1.46) | 0.40 | 1.14 (0.93–1.41) | 0.21 |
| AR Ratio | 2.01 (1.37–2.96) | <0.001 | 2.83 (1.81–4.40) | <0.001 | 2.38 (1.76–3.23) | <0.001 |
| CMV Pneumonitis | 1.03 (0.33–3.25) | 0.96 | 1.34 (1.00–1.80) | 0.05 | 1.10 (0.84–1.46) | 0.48 |
| Pseudomonas | 2.23 (1.56–3.19) | <0.001 | 1.02 (0.74–1.39) | 0.92 | 1.38 (1.09–1.74) | 0.007 |
| CARV | 1.50 (0.84–2.67) | 0.17 | 1.21 (0.82–1.80) | 0.34 | 1.27 (0.92–1.76) | 0.15 |
| Small conidia Asp sp | 1.69 (1.17–2.45) | 0.006 | 1.41 (1.05–1.90) | 0.02 | 1.53 (1.22–1.93) | <0.001 |
| Large conidia Asp sp | 1.54 (0.95–2.52) | 0.08 | 0.97 (0.70–1.33) | 0.83 | 1.03 (0.79–1.34) | 0.84 |
| Multivariable Model | ||||||
| Age ≥ 65 | 0.74 (0.46–1.19) | 0.21 | 1.48 (0.99–2.21) | 0.06 | 1.09 (0.80–1.47) | 0.60 |
| CF/bronchiectasis | 0.44 (0.19–0.99) | 0.05 | 0.85 (0.59–1.22) | 0.38 | 0.76 (0.55–1.06) | 0.10 |
| Single Lung Txp | 1.51 (1.00–2.28) | 0.05 | 1.74 (1.20–2.51) | 0.004 | 1.59 (1.21–2.09) | <0.001 |
| AR Ratio | 1.97 (1.29–3.01) | 0.002 | 2.69 (1.72–4.20) | <0.001 | 2.31 (1.72–3.11) | <0.001 |
| CMV Pneumonitis | 0.91 (0.28–2.95) | 0.88 | 1.20 (0.89–1.62) | 0.24 | 1.21 (0.91–1.61) | 0.19 |
| Pseudomonas | 2.20 (1.49–3.23) | <0.001 | 1.14 (0.82–1.59) | 0.43 | 1.40 (1.10–1.78) | 0.006 |
| Small conidia Asp sp | 1.54 (1.05–2.26) | 0.02 | 1.36 (1.01–1.83) | 0.04 | 1.44 (1.14–1.82) | 0.002 |
| Large conidia Asp sp | 1.13 (0.68–1.90) | 0.64 | 0.94 (0.68–1.30) | 0.70 | 1.01 (0.77–1.32) | 0.95 |
Joined cohort multivariable model also adjusted for center.
Abbreviations: CF: cystic fibrosis. Txp: transplant. ATG: anti-thymocyte globulin. AR: acute rejection. CMV: cytomegalovirus. CARV: community acquired respiratory virus. Asp sp: Aspergillus species.
In the multivariable models, colonization with small conidia Aspergillus species remained a significant risk factor for BOS at UCLA, DUMC, and in the joined cohort (P = 0.002, adjusted HR 1.44, 95% CI 1.14–1.82) (Table 4). Conversely, colonization with a large conidia Aspergillus species was not associated with BOS in either center or the joined cohort. In addition, the AR ratio and single lung transplant were associated with the risk of BOS in each center and the joined cohort, and Pseudomonas was a risk factor for BOS at UCLA and the joined cohort, but not at DUMC. We explored for interactions between variables, but these terms were not significant and not included in the final model.
Colonization with Small Conidia Aspergillus Species is Associated with Survival
In univariable analyses, colonization with small conidia Aspergillus species was significantly associated with an increased risk of death in each center, and in the joined cohort (P < 0.001, unadjusted HR 1.81, 95% CI 1.45–2.27) (Table 5). Colonization with large conidia Aspergillus species was not associated with mortality in either center. Other potential risk factors for death (P ≤ 0.10) in at least one center or the joined cohort included age ≥65 years, CF/bronchiectasis (negative association), single lung transplant, AR ratio, BOS, CMV pneumonitis, Pseudomonas infection, and CARV infection. Each of these variables was included in the final multivariable models.
TABLE 5.
COX MODELS FOR RISK OF MORTALITY
| UCLA Cohort |
DUMC Cohort |
Joined Cohort* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Univariable Models | ||||||
| Female Gender | 0.80 (0.56–1.15) | 0.23 | 1.06 (0.80–1.39) | 0.70 | 0.95 (0.76–1.18) | 0.65 |
| Age ≥ 65 | 1.50 (1.01–2.23) | 0.04 | 1.27 (0.83–1.93) | 0.27 | 1.46 (1.1–1.93) | 0.009 |
| Diagnosis | ||||||
| Restrictive/other | Reference | Reference | Reference | |||
| Obstructive | 1.14 (0.80–1.63) | 0.47 | 0.86 (0.64–1.17) | 0.35 | 0.92 (0.73–1.16) | 0.50 |
| CF/bronchiectasis | 0.21 (0.05–0.87) | 0.03 | 0.88 (0.59–1.30) | 0.51 | 0.71 (0.50–1.02) | 0.06 |
| Single Lung Txp | 1.36 (0.95–1.93) | 0.09 | 1.63 (1.15–2.30) | 0.006 | 1.62 (1.28–2.05) | <0.001 |
| ATG Induction | 1.17 (0.8–1.70) | 0.42 | 1.06 (0.80–1.39) | 0.70 | 1.15 (0.93–1.43) | 0.20 |
| AR Ratio | 1.36 (0.95–1.93) | 0.13 | 1.77 (1.17–2.67) | 0.007 | 1.51 (1.13–2.02) | 0.006 |
| BOS | 5.07 (3.45–7.44) | <0.001 | 8.84 (6.50–12.1) | <0.001 | 7.21 (5.67–9.16) | <0.001 |
| CMV Pneumonitis | 1.10 (0.35–3.48( | 0.87 | 1.64 (1.23–2.19) | <0.001 | 1.36 (1.04–1.76) | 0.02 |
| Pseudomonas | 2.01 (1.40–2.88) | <0.001 | 1.63 (1.22–2.18) | 0.001 | 1.79 (1.43–2.24) | <0.001 |
| CARV | 2.04 (1.22–3.42) | 0.007 | 1.53 (1.06–2.21) | 0.02 | 1.64 (1.22–2.21) | 0.001 |
| Small conidia Asp sp | 1.79 (1.25–2.56) | 0.002 | 1.77 (1.33–2.35) | <0.001 | 1.81 (1.45–2.27) | <0.001 |
| Large conidia Asp sp | 1.29 (0.81–2.07) | 0.29 | 1.11 (0.82–1.51) | 0.51 | 1.11 (0.86–1.44) | 0.42 |
| Multivariable Model | ||||||
| Age ≥ 65 | 1.32 (0.84–2.06) | 0.23 | 1.08 (0.70–1.67) | 0.72 | 1.22 (0.91–1.64) | 0.19 |
| CF/bronchiectasis | 0.27 (0.07–1.11) | 0.07 | 1.17 (0.81–1.68) | 0.40 | 0.99 (0.70–1.39) | 0.93 |
| Single Lung Txp | 1.17 (0.78–1.77) | 0.45 | 1.13 (0.78–1.63) | 0.53 | 1.22 (0.93–1.58) | 0.15 |
| AR Ratio | 1.02 (0.65–1.58) | 0.94 | 0.93 (0.63–1.37) | 0.73 | 0.95 (0.72–1.26) | 0.72 |
| BOS | 4.24 (2.93–6.55) | <0.001 | 8.50 (6.16–11.7) | <0.001 | 6.49 (5.06–8.34) | <0.001 |
| CMV Pneumonitis | 1.09 (0.33–3.60) | 0.89 | 1.50 (1.11–2.02) | 0.009 | 1.49 (1.12–1.98) | 0.007 |
| Pseudomonas | 1.54 (1.05–2.28) | 0.03 | 1.68 (1.24–2.27) | <0.001 | 1.59 (1.26–2.02) | <0.001 |
| CARV | 1.93 (1.14–3.26) | 0.01 | 1.34 (0.92–1.94) | 0.13 | 1.55 (1.14–2.09) | 0.005 |
| Small conidia Asp sp | 1.24 (0.85–1.81) | 0.27 | 1.30 (0.96–1.75) | 0.09 | 1.30 (1.03–1.64) | 0.03 |
| Large conidia Asp sp | 0.97 (0.59–1.59) | 0.90 | 0.82 (0.60–1.13) | 0.23 | 0.87 (0.66–1.13) | 0.28 |
Joined cohort multivariable model also adjusted for center.
Abbreviations: CF: cystic fibrosis. Txp: transplant. ATG: anti-thymocyte globulin. AR: acute rejection. BOS: bronchiolitis obliterans syndrome. CMV: cytomegalovirus. CARV: community acquired respiratory virus. Asp sp: Aspergillus species.
In the multivariable models, colonization with small conidia Aspergillus species remained a significant risk factor for mortality in the joined cohort (P = 0.03, HR 1.30, 95% CI 1.03–1.64) (Table 5). Colonization with large conidia Aspergillus species was not associated with death in either center. In addition, BOS and Pseudomonas infection were associated with mortality in each center and the joined cohort. CMV pneumonitis was a risk factor for death at DUMC and in the joined cohort, while CARV infection was a risk factor for death at UCLA and in the joined cohort.
DISCUSSION
Aspergillus is one of the most common pathogens isolated from respiratory secretions of lung transplant patients. Historically, Aspergillus colonization without signs of tissue invasion was thought to be less consequential, with the main concern being an increased risk of subsequent invasive disease. However, in a previous single center study at UCLA, we reported that Aspergillus colonization, like other common respiratory infections after lung transplantation, is associated with an increased risk for BOS (14). BOS is the most important survival limiting condition following lung transplantation. In our previous study, all species of Aspergillus were considered in aggregate. However, each Aspergillus species has unique traits and virulence factors, and the various Aspergillus species may behave differently in terms of their relationship with BOS.
In the current study, using two large cohorts, we focused on conidia diameter, a trait that differs across Aspergillus species and may determine the probability that colonization occurs in small airways. We demonstrate that colonization with both small and large conidia Aspergillus species is common among lung transplant recipients from two transplant centers on opposite coasts of the United States. While the incidence of colonization with small conidia Aspergillus species was similar between centers, we do find a higher incidence of colonization with large conidia species at DUMC compared to UCLA. In part, this may be explained by geographic heterogeneity of A. niger and other large conidia species (21). In contrast, A. fumigatus, the dominant small conidia species, has no geographic predilection (21).
Similar to our previous work, our statistical methods recognize and account for the time-dependent nature of Aspergillus colonization as a risk factor for BOS. We considered colonization occurring at any time point post-transplantation and before a diagnosis of BOS as a time-dependent variable in both unadjusted and adjusted models. Likewise, we also explored Aspergillus colonization at any time post-transplantation as a time-dependent variable in models of survival. We also recognize that death is a competing risk for both Aspergillus colonization and BOS, with potential important implications on the results. For this reason, competing risk analyses were also performed using cause specific Cox regression models for both the colonization and BOS outcomes. These models yielded virtually identical results (data not shown) as the standard Cox models. Collectively, these approaches provide compelling evidence that Aspergillus colonization with small conidia species, but not large conidia species, increases the risk of BOS and mortality after lung transplantation.
Although not the primary aim of this study, we also evaluated for other potential risk factors of BOS and mortality. Consistent with the existing literature, we did find that AR (modeled as the AR ratio) was strongly associated with the risk of BOS, and that BOS has a major impact on the risk of death following lung transplantation. We also found that single lung transplant was a consistent risk factor for BOS, even after adjustment for other variables. We speculate that a lower baseline FEV1 in single lung recipients may be a confounder in this association, as has been described (25). In univariable analyses, CMV pneumonitis was a risk factor for BOS and death at DUMC, consistent with prior work (9). The relationship with BOS was not present after multivariable adjustment, but the relationship with mortality persisted. CMV pneumonitis at UCLA occurred too infrequently to show an association with outcomes. Pseudomonas infection was a strong risk factor for BOS at UCLA, but not at DUMC. The explanations for this disparate finding are not apparent, but differences in patient populations may play a role. In one report, only de novo Pseudomonas infection post transplant was associated with BOS, while those positive for Pseudomonas pretransplant had no increased risk (10). We did not collect pretransplant infection data in this study. Pseudomonas was a risk factor for death in each center. CARV infection was not significantly associated with BOS in either center, contradicting prior reports (11, 13). However, in our study we did not determine whether CARV infections were associated with lower respiratory tract symptoms, which is reported to be important in determining the risk of BOS (11). CARV diagnostics were also only limited to antigen detection and culture. Given the much higher sensitivity of PCR diagnostics, it is conceivable that a number of viral infections were merely not diagnosed. CARV infection was a risk factor for death at each center in univariable analyses, and CARV remained a predictor of mortality at UCLA and in the joined cohort after multivariable adjustment.
As compared with prior reports, a unique strength of this study is that our findings are validated across two large cohorts spanning approximately 10 years. Given the paucity of prospective, multi-center studies in the field, the need to replicate and validate retrospective, single-center findings is critical. Moreover, our findings were validated in two centers that differ in numerous clinical practice and patient selection aspects, suggesting that our findings may be generalizable to other centers. For example, significant differences between cohorts included the proportion of single vs. bilateral lung transplant operations, incidence of CMV pneumonitis, and the burden of AR, all considered putative risk factors for BOS. Some differences were also related to the risk of colonization with either large or small conidia species. Specifically, bilateral lung transplantation was associated with an increased risk of colonization with large conidia Aspergillus, possibly due to the presence of two large airway anastomoses. Older age (≥65 years) at the time of transplantation was associated with an increased risk of colonization with small conidia Aspergillus species, a characteristic that was more common at UCLA.
There are two plausible, albeit disparate, explanations for a relationship between Aspergillus colonization and BOS. The first is that Aspergillus colonization is an epiphenomenon of the pathogenesis of OB. In this scenario, airways pathology and/or treatments (eg. augmented immune suppression) directed at this pathology create an environment favorable for Aspergillus colonization. The second, alternate explanation is that Aspergillus colonization can promote OB pathogenesis. We recognize that our study design cannot definitively answer the question as to which explanation is correct. However, if a causative relationship does exist, then the anatomic site of Aspergillus colonization must include the small airways. An inhaled particle's diameter is known to influence the site of deposition within the airways (16–19). This property has been exploited by the pharmaceutical industry seeking to maximize delivery of inhaled drugs to an affected area of the lung (i.e. the small airways in asthma) (18). Particles with a diameter in the 2–3μm range are ideal for delivery and deposition in the small airways (19). Importantly, we find that only colonization with those Aspergillus species' most suited to reach and colonize the small airways is associated with an increased risk of BOS. We acknowledge that conidia size is only one of many traits that differ between Aspergillus species including adhesion to respiratory epithelium, resistance to phagocytosis, germination rates, thermotolerance, cell wall composition and structure, and elaboration of enzymes (26). Notably, our small conidia Aspergillus group was dominated by a single species, A. fumigatus. Findings when A. fumigatus was considered alone (data not shown) were similar to those of the group of small conidia species that included A. fumigatus. The sample size of non-fumigatus small conidia species was too small to examine separately. Therefore, it is possible that a trait unique to A. fumigatus, other than conidia size, may be responsible for the association with BOS.
Despite the strengths of our study, several other potential limitations exist. First, as with any retrospective study, potential sampling biases exist. In this study, patients diagnosed with BOS had one more bronchoscopy on average at UCLA (5.0 ± 2.0 vs. 4.0 ± 1.7) and 2 fewer bronchoscopies on average at DUMC (8.7 ± 4.9 vs. 10.6 ± 5.0). However, we would expect that a sampling bias would affect small and large conidia species of Aspergillus equally, and therefore this bias could not explain the differences in risk of BOS. It might be useful to assess the impact of other non-Aspergillus molds on BOS based upon spore size, but isolation of these molds were too infrequent at each center to assess in this study. Furthermore, our analysis could not consider all reported risk factors for BOS. For example, primary graft dysfunction (PGD) is a reported risk factor for BOS, but PGD grades were not collected in this study. Finally, in this study we did not differentiate between Aspergillus colonization and invasive disease. In our prior study, all diagnoses of invasive Aspergillosis were preceded by Aspergillus colonization or occurred after a diagnosis of BOS. Therefore, the impact of invasive disease on our findings is probably negligible.
Given the known consequences of invasive Aspergillosis, many lung transplant centers, including both in this study, have used short-term antifungal prophylaxis strategies to limit the risk of invasive disease. After the bronchial anastomoses have healed the risk of invasive disease declines, but colonization remains common. We have demonstrated that colonization of the lung allograft with small conidia Aspergillus species, but not large conidia species, is associated with an increased risk of BOS. This finding suggests a possible mechanism, where colonization of the allograft small airways is required for Aspergillus to impact the development of BOS. Importantly, compared with our initial single center observation of an association between Aspergillus and BOS, this study included as validation, a second large lung transplant center with a different patient population and different clinical protocols. These findings now warrant prospective evaluation of prophylaxis strategies targeting Aspergillus colonization of the small allograft airways as well as invasive disease, with the goal of preserving lung allograft function as long as possible.
ACKNOWLEDGEMENTS
Funding: Supported by K23 HL094746 (S.S.W.); K24 091140-01 (S.M.P.); KL2 RR024127 (L.D.S.); R01 HL112990-01 (J.A.B.), and the National Center for Research Resources, Grant UL1 RR033176, and is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000124 (S.M. Dubinett).
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- OB
obliterative bronchiolitis
- AR
acute rejection
- CMV
cytomegalovirus
- CARV
community acquired respiratory virus
- UCLA
University of California, Los Angeles
- DUMC
Duke University Medical Center
- PFT
pulmonary function test
- ATG
anti-thymocyteglobulin
- TBBX
transbronchial biopsy
- PGD
primary graft dysfunction.
Footnotes
DISCLOSURE The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26(7):681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 5.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6(1):108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 6.Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 31(2):189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 182;6(784):789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 181(12):1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, et al. Pseudomonas aeruginosa Colonization of the Allograft After Lung Transplantation and the Risk of Bronchiolitis Obliterans Syndrome. Transplantation. 2008;85(5):771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 11.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 12.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM. Pseudomonal airway colonization: a risk factor for BOS after lung transplantation? Eur Respir J. 2008 doi: 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(8):1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol. 150(4):406–417. doi: 10.1111/j.1365-2141.2010.08283.x. [DOI] [PubMed] [Google Scholar]
- 16.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1(4):315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 17.Heyder J GJ, Rudolf G, Schiller CF, Stahlhofen W. Deposition of Particles in the Human Respiratory Tract in the Size Range 0.005-15 μm. J Aerosol Sci. 1986;17(5):811–825. [Google Scholar]
- 18.Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172(12):1497–1504. doi: 10.1164/rccm.200410-1414OC. [DOI] [PubMed] [Google Scholar]
- 19.Gerrity TR, Lee PS, Hass FJ, Marinelli A, Werner P, Lourenco RV. Calculated deposition of inhaled particles in the airway generations of normal subjects. J Appl Physiol. 1979;47(4):867–873. doi: 10.1152/jappl.1979.47.4.867. [DOI] [PubMed] [Google Scholar]
- 20.Weigt SS, Finlen Copeland CA, Derhovanessian A, Davis WA, Snyder LD, Ross DJ, et al. A Multi-Center Validation Of Pulmonary Aspergillus Fumigatus Colonization As A Risk Factor For Bronchiolitis Obliterans Syndrome After Lung Transplantation [abstract] Am J Respir Crit Care Med. 2011;183(A4020) [Google Scholar]
- 21.Hartwig M, Snyder L, Finlen-Copeland C, Lin S, Zaas D, Davis R, et al. Lung transplantation at Duke University. Clinical Transplants 01/2009. [PubMed] [Google Scholar]
- 22.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanev S. Aspergillus and Penicillium. CBS Fungal Biodiversity Centre. 2011 [Google Scholar]
- 24.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. The Journal of Heart and Lung Transplantation. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 25.Burton CM, Iversen M, Mortensen J, Carlsen J, Andersen CB, Milman N, et al. Post-transplant baseline FEV1 and the development of bronchiolitis obliterans syndrome: an important confounder? J Heart Lung Transplant. 2007;26(11):1127–1134. doi: 10.1016/j.healun.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Pasqualotto AC. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med Mycol. 2009;47(Suppl 1):S261–270. doi: 10.1080/13693780802247702. [DOI] [PubMed] [Google Scholar]