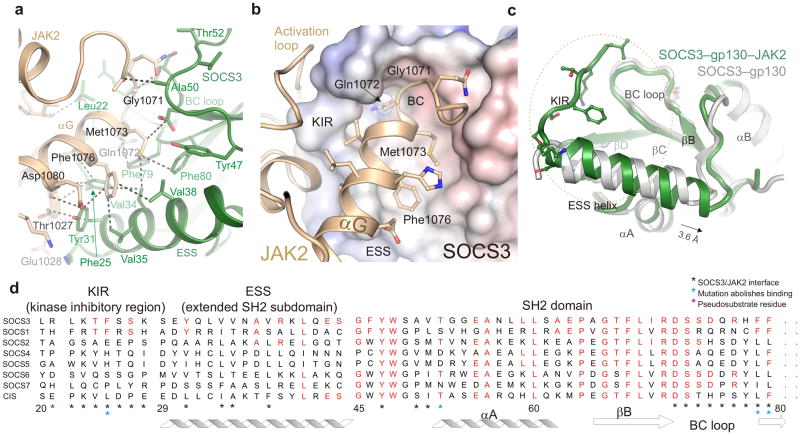
Figure 2. The SOCS3/JAK2 interaction.
(a) The hydrophobic SOCS3/JAK2 interface. Important residues are labeled and a selection of van der Waals contacts shown as dotted lines. Color scheme is the same as Figure 1. (b) Residues from the GQM motif and αG28 helix of JAK2 bind a concave hydrophobic surface on SOCS3 formed by the KIR, BC loop and extended SH2 subdomain (ESS). JAK2 is shown in ribbon representation and SOCS3 as an electrostatic surface (+/− 250mV). The GQM motif and Phe1076 from αG of JAK2 are highlighted. (c) Comparison of the SOCS3 structure in isolation (PDB:2HMH26, white) and in complex with JAK2 (this work, green). The ESS helix can be seen to undergo a translation of half a helical turn upon binding JAK2 whilst the KIR (which is unstructured in the absence of JAK2) adopts an extended structure. The orientation of SOCS3 is the same as in (b). (d) Sequence conservation of the JAK2-binding interaction surface between SOCS1 and SOCS3. Conserved residues are shown in red.