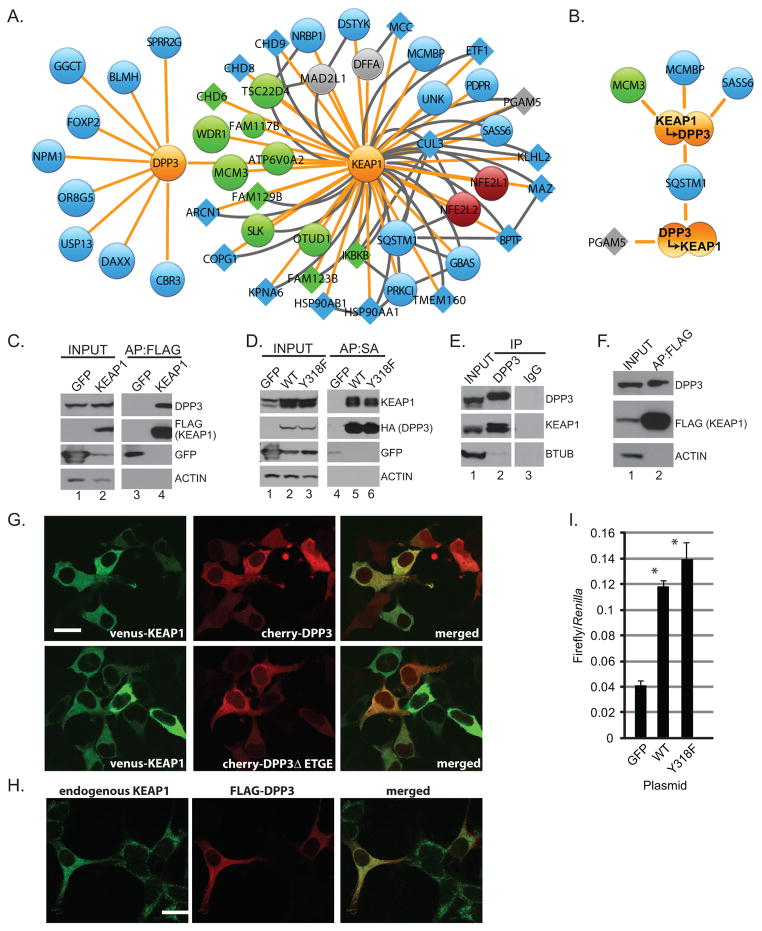
Figure 3. DPP3 is a KEAP1 interacting protein.
(A) Schematic protein interaction network for DPP3 and KEAP1. Node and edge coloring and sizing are consistent with Figure 1. (B) HEK293T cells stably expressing KEAP1 and DPP3 were lysed and subjected to two sequential rounds of affinity purification before mass spectrometry. Data shown represent biological duplicate experiments, wherein the order of affinity purifications was reversed. (C) Protein complexes from HEK293T cells stably expressing FLAG-KEAP1 were affinity purified and analyzed by Western blot. (D) Protein complexes from HEK293T cells stably expressing SBPHA-DPP3 or SBPHA-DPP3-Y318F were streptavidin affinity purified and analyzed by Western blot. (E) Endogenous DPP3 from HEK293T cells was immunopurified and analyzed by Western blot for the indicated endogenous proteins. (F) Protein complexes were FLAG affinity purified from the lung adenocarcinoma cell line H2228 stably expressing FLAG-KEAP1 and analyzed by Western blot. (G) HEK293 cells were transfected with venus-KEAP1 and the indicated mCherry-fused DPP3 expression construct. (H) HEK293T cells transfected with FLAG-DPP3 and stained for FLAG and endogenous KEAP1. Scale =20 μm. (I) HEK293T cells were transfected with NQO1-ARE-luciferase, constitutively active Renilla luciferase and the indicated expression plasmid before lysis and luciferase quantification (* P<0.05 across three biological replicate experiments).