Figure 1.
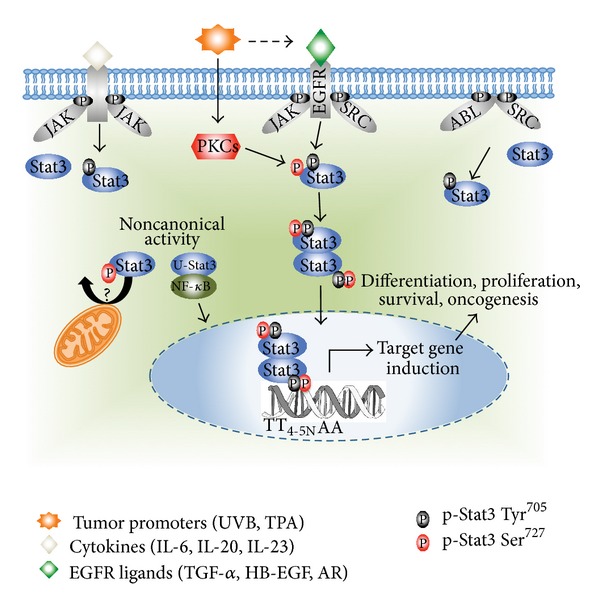
Pathways associated with Stat3 activation. Stat3 is activated downstream of receptor tyrosine kinases (e.g., EGFR), cytokine receptors via associated Janus family kinases (JAKs) (e.g., IL-6 receptor), and nonreceptor-associated tyrosine kinases (e.g., c-src). Tumor promoters such as TPA and UVB activate Stat3 in keratinocytes primarily via the EGFR. Activation of PKCs by tumor promoters leads to the processing of membrane-bound proforms of EGFR ligands such as heparin-binding EGF (HB-EGF) by matrix metalloproteinases (MMPs). In addition, PKCs associate with and phosphorylate Stat3 at Ser727, which is necessary for maximal Stat3 transcriptional activity. Furthermore, transcriptional induction of cytokines and EGF ligands can lead to autocrine stimulation and sustained Stat3 phosphorylation. After phosphorylation, STAT3 dimerizes and translocates to the nucleus, where Stat3 dimers directly regulate gene expression of transcriptional targets including Bcl-xL, cyclin D1, c-myc, Twist and Survivin. STAT3-mediated regulation of target gene expression is involved in various cellular functions including cell differentiation, proliferation, survival, and oncogenesis. Stat3 can also act through noncanonical signaling pathways. In this regard, unphosphorylated Stat3 (U-Stat3) can drive gene expression of a subset of genes that are different from p-Stat3 dimers in an NF-κB-dependent and independent manner. In addition, p-Stat3 Ser727 can translocate into the mitochondria and influence mitochondrial respiratory chain activity. These noncanonical Stat3 signaling pathways have protumorigenic roles in certain cell/tissue types; however their role in epithelial carcinogenesis has not been evaluated.
