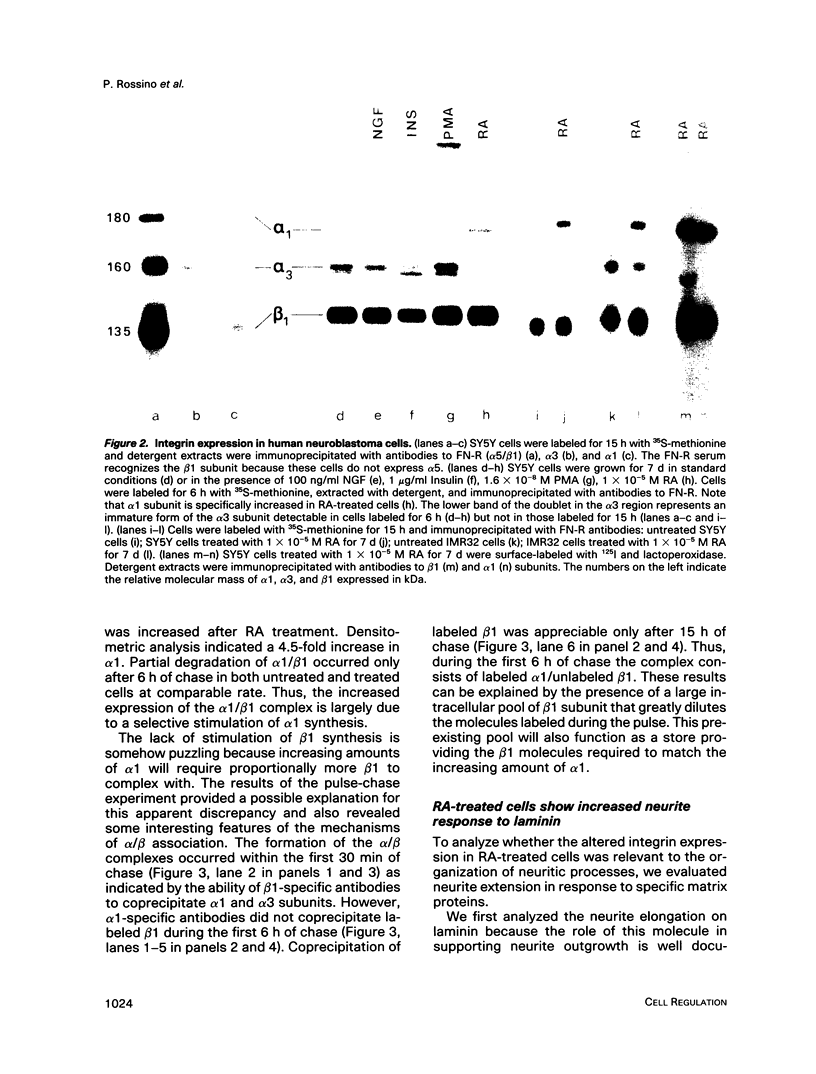
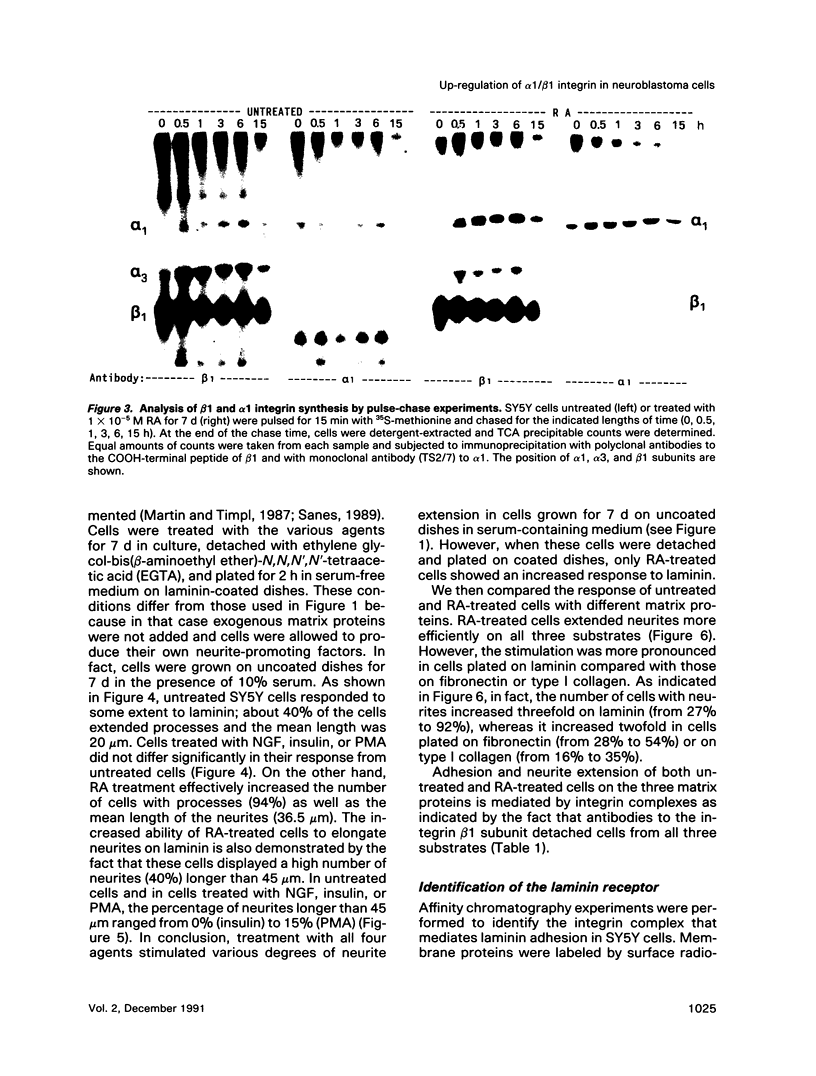
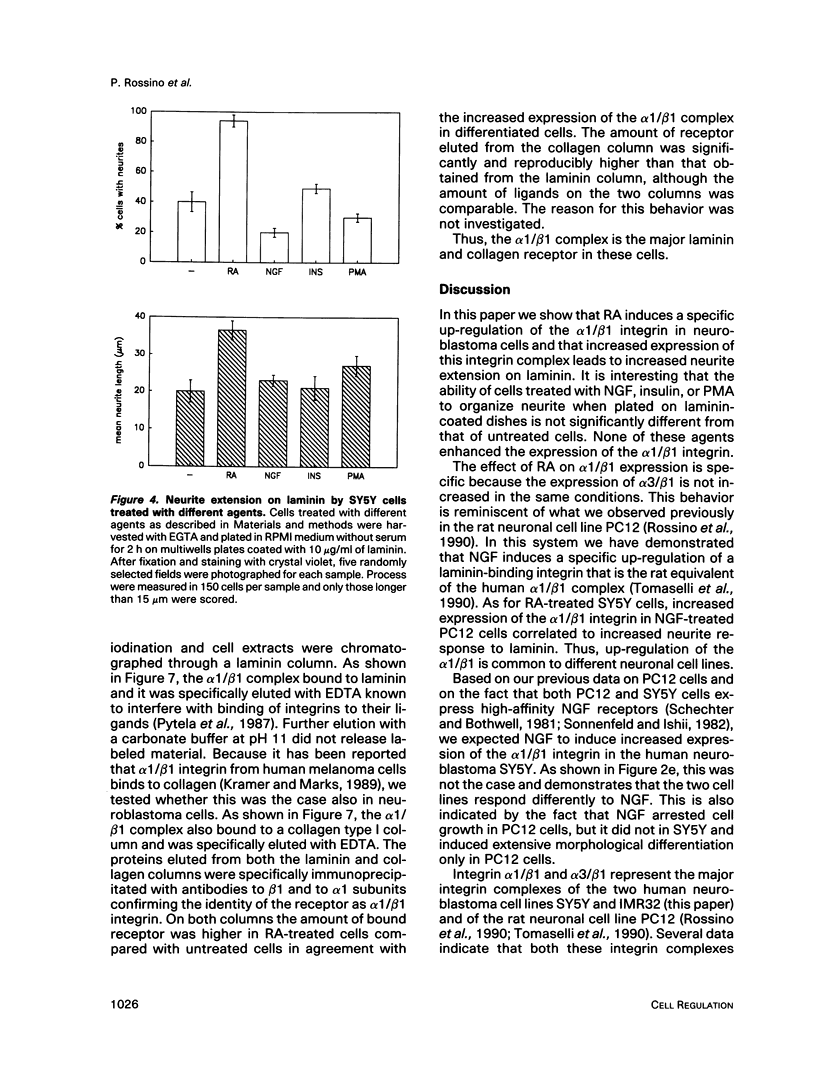
Abstract
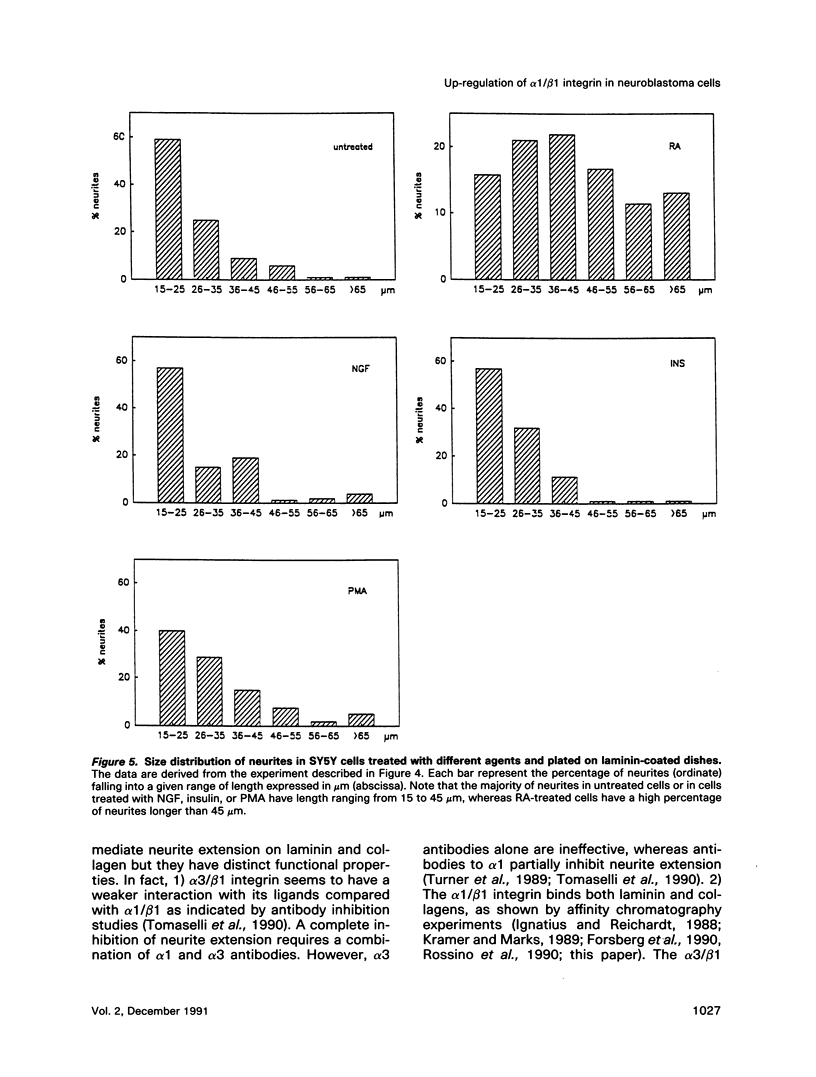
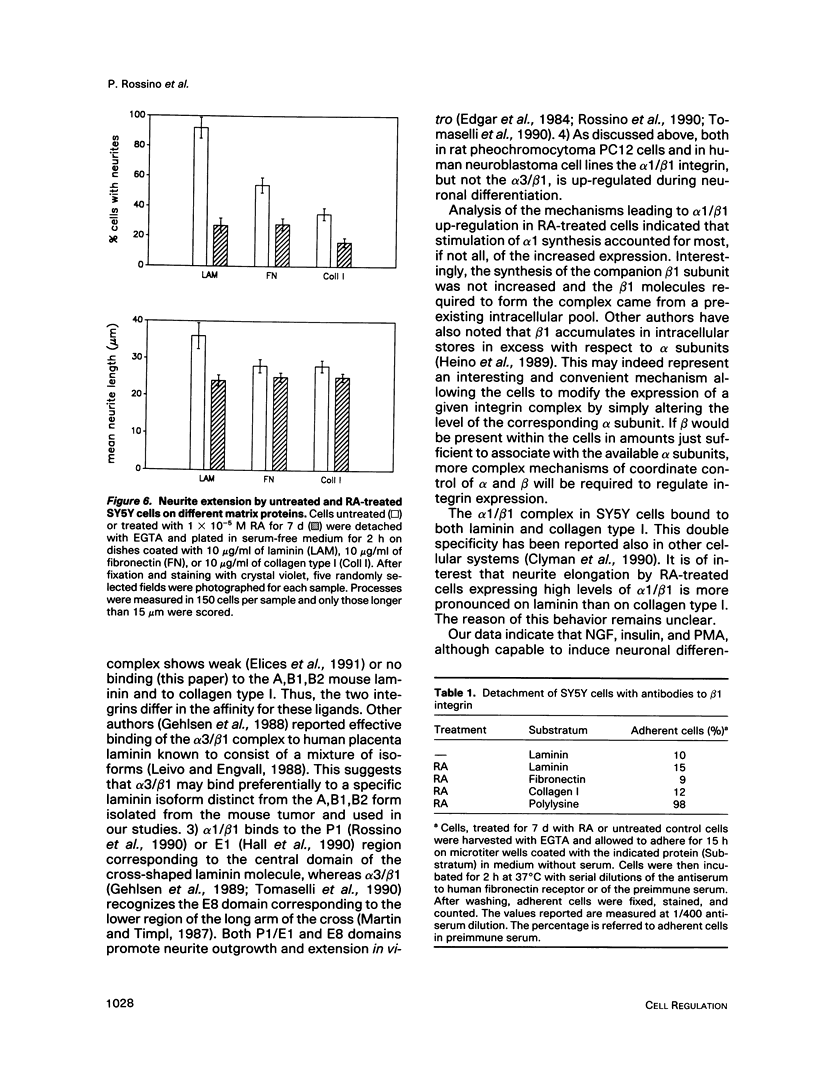
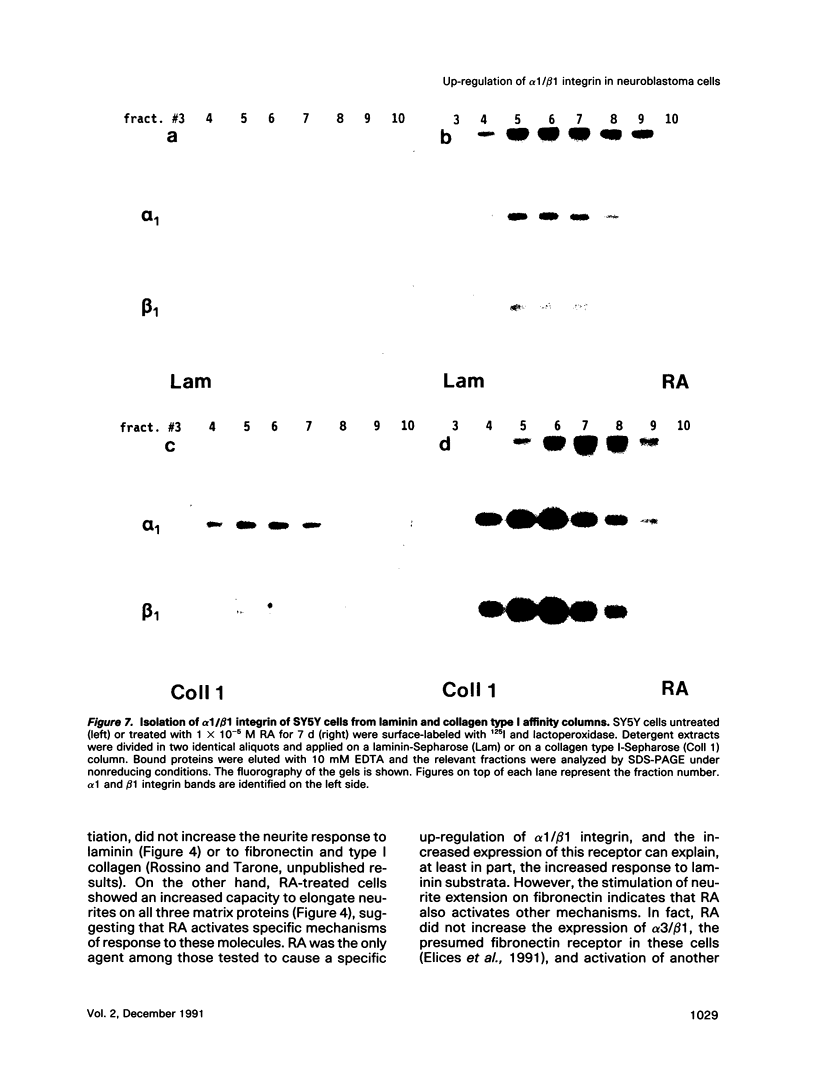
Retinoic acid (RA) is known to induce differentiation of neuroblastoma cells in vitro. Here we show that treatment of two human neuroblastoma cell lines, SY5Y and IMR32, with RA resulted in a fivefold increase of the integrin alpha 1/beta 1 expression. The effect was selective because expression of the alpha 3/beta 1 integrin, also present in these cells, was not increased. The up-regulation of the alpha 1/beta 1 differentiated SY5Y cells correlated with increased neurite response to laminin. In fact, RA-treated SY5Y cells elongated neurites on laminin-coated substratum more efficiently compared with untreated cells or cells treated with nerve growth factor, insulin, or phorbol 12-myristate 13-acetate. These three agents induced partial morphological differentiation but did not increase alpha 1 integrin expression. Neurite extension in RA-treated cells was more efficient on laminin than on fibronectin or collagen type I and was inhibited with beta 1 integrin antibodies on all three substrates. Affinity chromatography experiments showed that alpha 1/beta 1 is the major laminin receptor in both untreated and RA-treated SY5Y cells. These data show that RA, a naturally occurring morphogen implicated in embryonic development, can selectively regulate the expression of integrin complexes in neuronal cells and suggest an important role of the alpha 1/beta 1 laminin receptor in the morphological differentiation of nerve cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adem A., Mattsson M. E., Nordberg A., Påhlman S. Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: regulation by phorbol ester and retinoic acid-induced differentiation. Brain Res. 1987 Jun;430(2):235–242. doi: 10.1016/0165-3806(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Biedler J. L., Roffler-Tarlov S., Schachner M., Freedman L. S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978 Nov;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Bixby J. L., Lilien J., Reichardt L. F. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988 Jul;107(1):353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Pratt R. S., Lilien J., Reichardt L. F. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca2+-dependent and -independent cell adhesion molecules. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Kleitman N., Dean A. C. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev Neurosci. 1989;11(4-5):348–360. doi: 10.1159/000111911. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clementi F., Cabrini D., Gotti C., Sher E. Pharmacological characterization of cholinergic receptors in a human neuroblastoma cell line. J Neurochem. 1986 Jul;47(1):291–297. doi: 10.1111/j.1471-4159.1986.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Turner D. C., Kramer R. H. An alpha 1/beta 1-like integrin receptor on rat aortic smooth muscle cells mediates adhesion to laminin and collagen types I and IV. Arteriosclerosis. 1990 May-Jun;10(3):402–409. doi: 10.1161/01.atv.10.3.402. [DOI] [PubMed] [Google Scholar]
- Conforti G., Zanetti A., Colella S., Abbadini M., Marchisio P. C., Pytela R., Giancotti F., Tarone G., Languino L. R., Dejana E. Interaction of fibronectin with cultured human endothelial cells: characterization of the specific receptor. Blood. 1989 May 1;73(6):1576–1585. [PubMed] [Google Scholar]
- Defilippi P., Truffa G., Stefanuto G., Altruda F., Silengo L., Tarone G. Tumor necrosis factor alpha and interferon gamma modulate the expression of the vitronectin receptor (integrin beta 3) in human endothelial cells. J Biol Chem. 1991 Apr 25;266(12):7638–7645. [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Urry L. A., Hemler M. E. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991 Jan;112(1):169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Forsberg E., Paulsson M., Timpl R., Johansson S. Characterization of a laminin receptor on rat hepatocytes. J Biol Chem. 1990 Apr 15;265(11):6376–6381. [PubMed] [Google Scholar]
- Gehlsen K. R., Dickerson K., Argraves W. S., Engvall E., Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminin. J Biol Chem. 1989 Nov 15;264(32):19034–19038. [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Ignatius M. J., Reichardt L. F. Identification of a neuronal laminin receptor: an Mr 200K/120K integrin heterodimer that binds laminin in a divalent cation-dependent manner. Neuron. 1988 Oct;1(8):713–725. doi: 10.1016/0896-6273(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Marks N. Identification of integrin collagen receptors on human melanoma cells. J Biol Chem. 1989 Mar 15;264(8):4684–4688. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lander A. D. Mechanisms by which molecules guide axons. Curr Opin Cell Biol. 1990 Oct;2(5):907–913. doi: 10.1016/0955-0674(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983 Mar;96(3):920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985 Oct;4(10):2505–2511. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M., Ong D. E., Chytil F. Retinoid-binding protein distribution in the developing mammalian nervous system. Development. 1990 May;109(1):75–80. doi: 10.1242/dev.109.1.75. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Momoi T., Hanaoka K., Momoi M. Spatial and temporal expression of cellular retinoic acid binding protein (CRABP) along the anteroposterior axis in the central nervous system of mouse embryos. Biochem Biophys Res Commun. 1990 Jun 29;169(3):991–996. doi: 10.1016/0006-291x(90)91992-2. [DOI] [PubMed] [Google Scholar]
- Neugebauer K. M., Tomaselli K. J., Lilien J., Reichardt L. F. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988 Sep;107(3):1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Polo J. R., Werbach-Perez K., Tiffany-Castiglioni E. A human clonal cell line model of differentiating neurons. Dev Biol. 1979 Aug;71(2):341–355. doi: 10.1016/0012-1606(79)90174-x. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Differentiation of neuroblastoma cells in culture. Biol Rev Camb Philos Soc. 1975 May;50(2):129–165. doi: 10.1111/j.1469-185x.1975.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Odelstad L., Larsson E., Grotte G., Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981 Nov 15;28(5):583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Ruusala A. I., Abrahamsson L., Mattsson M. E., Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984 Jun;14(2):135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Ishii D. N. Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984 Jun 8;302(2):323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- Rees J. L., Daly A. K., Redfern C. P. Differential expression of the alpha and beta retinoic acid receptors in tissues of the rat. Biochem J. 1989 May 1;259(3):917–919. doi: 10.1042/bj2590917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Bixby J. L., Hall D. E., Ignatius M. J., Neugebauer K. M., Tomaselli K. J. Integrins and cell adhesion molecules: neuronal receptors that regulate axon growth on extracellular matrices and cell surfaces. Dev Neurosci. 1989;11(4-5):332–347. doi: 10.1159/000111910. [DOI] [PubMed] [Google Scholar]
- Rossino P., Gavazzi I., Timpl R., Aumailley M., Abbadini M., Giancotti F., Silengo L., Marchisio P. C., Tarone G. Nerve growth factor induces increased expression of a laminin-binding integrin in rat pheochromocytoma PC12 cells. Exp Cell Res. 1990 Jul;189(1):100–108. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Bothwell M. A. Nerve growth factor receptors on PC12 cells: evidence for two receptor classes with differing cytoskeletal association. Cell. 1981 Jun;24(3):867–874. doi: 10.1016/0092-8674(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld K. H., Ishii D. N. Nerve growth factor effects and receptors in cultured human neuroblastoma cell lines. J Neurosci Res. 1982;8(2-3):375–391. doi: 10.1002/jnr.490080226. [DOI] [PubMed] [Google Scholar]
- Spinelli W., Sonnenfeld K. H., Ishii D. N. Effects of phorbol ester tumor promoters and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Cancer Res. 1982 Dec;42(12):5067–5073. [PubMed] [Google Scholar]
- Tarone G., Galetto G., Prat M., Comoglio P. M. Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol. 1982 Jul;94(1):179–186. doi: 10.1083/jcb.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Paulsson M., Dziadek M., Fujiwara S. Basement membranes. Methods Enzymol. 1987;145:363–391. doi: 10.1016/0076-6879(87)45021-0. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Hall D. E., Flier L. A., Gehlsen K. R., Turner D. C., Carbonetto S., Reichardt L. F. A neuronal cell line (PC12) expresses two beta 1-class integrins-alpha 1 beta 1 and alpha 3 beta 1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990 Nov;5(5):651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Neugebauer K. M., Bixby J. L., Lilien J., Reichardt L. F. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988 Mar;1(1):33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Turner D. C., Flier L. A., Carbonetto S. Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite outgrowth on laminin and collagen. J Neurosci. 1989 Sep;9(9):3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T. The plasma membrane as a regulatory site in growth and differentiation of neuroblastoma cells. Int Rev Cytol. 1982;74:1–54. doi: 10.1016/s0074-7696(08)61168-7. [DOI] [PubMed] [Google Scholar]