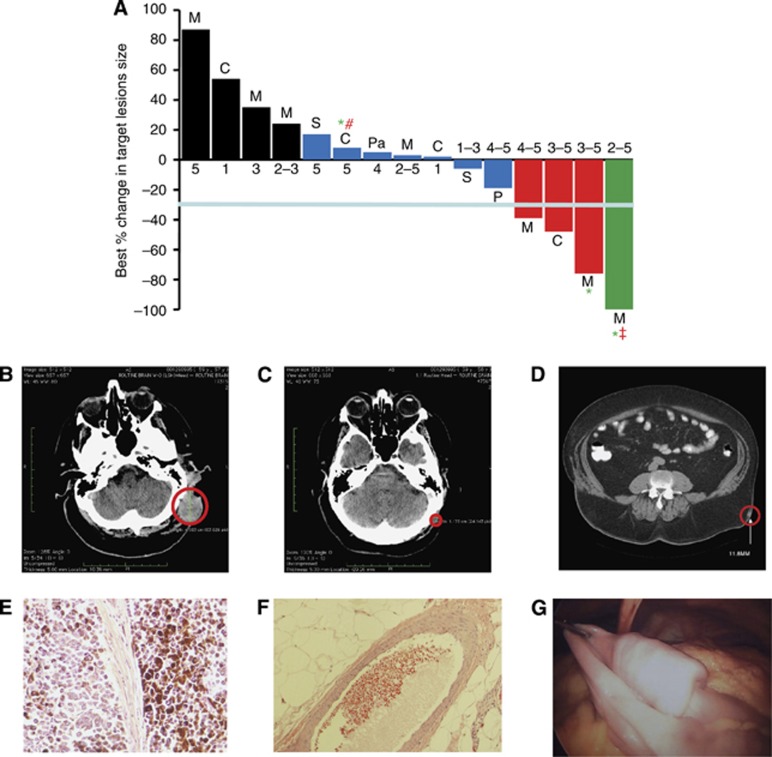
Figure 4.
Tumour Response to p28. (A) Best response (%) of target lesions by patient. Before study entry, all patients underwent physical examination, laboratory measurements, and computed tomography scans as baseline. Tumour lesions were accurately measured in at least one dimension (longest diameter) with a minimum size of 10 mm by CT scan. Tumour response and progression were evaluated by Response Evaluation Criteria in Solid Tumours (RECIST 1.1). Complete Response (CR) was defined as disappearance of all target lesions. Partial response (PR) was defined as a 30% or above decrease in the sum of the longest diameters of target lesions, excluding complete disappearance of disease. Progressive disease (PD) was defined as a 20% or above increase in the sum of the longest diameters of target lesions. Stable disease (SD) was defined as small changes that did not meet the criteria for a PR or PD. M=melanoma, C=colon, P=prostate, S=sarcoma and Pa=pancreatic cancer. CR=green, PR=red, SD=blue, PD=black. *, Alive. All patients with tumour reduction evaluated as stable, PR and CR received either the highest or multiple levels of p28 (RECIST guideline, version 1.1; Eisenhauer et al, 2009). Values below each bar indicate the dose level (range) each patient received. #, progressive disease 28 December 2011, ‡, Cancer recurrence 23 July 2012. (B,C) Representative CT images of the head region (B) prior (baseline) and (C) post trial completion (dose level 3–5). (D–F) Another subject receiving dose levels 2–5 during the trial showed complete regression of an intramuscular nodule (D), red circle=target lesion; histological examinations of target lesion in left flank before p28 administration (E) and after receiving dose levels 2–5 during the trial (F). (G) An image of a polypoid metastatic lesion in the proximal ilium that recurred after completion of the study.