Abstract
Adenosine, a purine nucleoside generated by the dephosphorylation of adenine nucleotides, is a potent endogenous physiologic and pharmacologic regulator of many functions. Adenosine was first reported to inhibit the inflammatory actions of neutrophils nearly 30 years ago and since then the role of adenosine and its receptors as feedback regulators of inflammation has been well established. Here we review the effects of adenosine, acting at its receptors, on neutrophil and monocyte/macrophage function in inflammation. Moreover, we review the role of adenosine in mediating the anti-inflammatory effects of methotrexate, the anchor drug in the treatment of Rheumatoid Arthritis and other inflammatory disorders.
Keywords: monocytes, macrophages, adenosine, adenosine receptors, neutrophils
Introduction
The nucleoside adenosine is a potent physiologic and pharmacologic regulator that is produced by cells in response to stress by breakdown of adenosine triphosphate (ATP) (Hasko and Cronstein, 2004; Sitkovsky et al., 2004; Hasko et al., 2005, 2008). ATP is broken down both intracellularly and extracellularly to generate adenosine (Figure 1). Intracellular adenosine is exported from cells via equilibrative nucleoside transporters or during apoptosis or necrosis. ATP and its degradation product adenosine diphosphate (ADP) are released from cells through a variety of mechanisms, including membrane damage, through connexin/pannexin and other channels, and through protein or hormone-transporting vesicles. When ATP and ADP are released, the phosphate groups of extracellular ATP and ADP are sequentially hydrolyzed, first by ecto-nucleoside triphosphate diphosphorylases (NTPDases, including CD39) and then by ecto-5′-nucleotidase (Ecto-5′NTase, CD73) (Yegutkin, 2008).
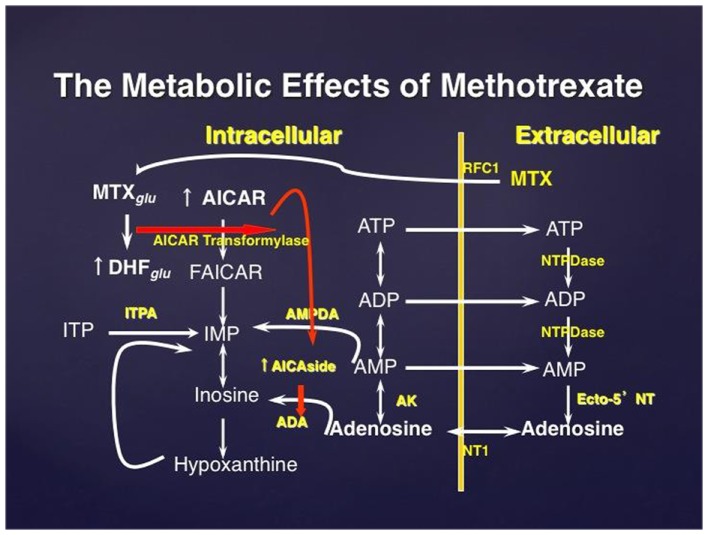
Figure 1.
The effect of methotrexate on adenosine release. Methotrexate is actively transported into the cell where it is polyglutamated; MTX polyglutamate is a potent inhibitor of AMP deaminase. Accumulation of AICAR, an intermediate metabolite in de novo purine biosynthesis, leads to enhanced release of adenine nucleotides which are released into the extracellular space and converted to adenosine. MTXglu, methotrexate polyglutamates; DHFglu, dihydrofolate polyglutamates; AICAR, aminoimidazole carboxamidoribonucleotide; FAICAR, formyl AICAR; RFC1, reverse folate carrier 1; ADA, adenosine deaminase; AK, adenosine kinase; NTPDase, nucleoside triphosphate phosphohydrolase; ecto-5′NT, ecto-5′nucleotidase.
Extracellular levels of adenosine can rise from low nano-molar to micro-molar concentrations in response to stress (Hasko et al., 2008). Adenosine regulates cell function through ligation of adenosine receptors, which consist of a family of four cell surface 7-transmembrane receptors (A1R, A2AR, A2BR, and A3) (Linden, 2001). The activation of A1 and A3 receptors leads to decreased intracellular cyclic adenosine monophosphate (Gallardo-Soler et al., 2008) levels by coupling to pertussis toxin-inhibited Gi-coupled signal transduction proteins. A2A receptors are GαS- or Gαolf-linked receptors that activate adenylyl cyclase, increase cAMP, and activate protein kinase A (PKA) and Epac1/2which activate their own signaling cascades to regulate cellular function. Interestingly, A2A receptors can also signal in a G protein-independent manner. A2B receptors can signal through both GαS and Gq proteins.
Adenosine and the Signs of Inflammation
Classically, inflammation is characterized by rubor (redness), tumor (swelling), calor (heat), and functio laesa (loss of function). These manifestations of inflammation result principally from vascular dilatation and leakage and, although a large number and variety of mediators are involved in inflammation it is likely that adenosine, released at sites of tissue injury, plays a role in the pathogenesis or regulation of these signs. Adenosine, acting primarily at A2A receptors, has long been known to be a potent vasodilator (Drury and Szent-Gyorgi, 1929) and this is the basis for use of adenosine and adenosine A2A receptor agonists for pharmacologic stress testing. Thus, it is likely adenosine release at inflamed sites contributes to the erythema (rubor) and resulting heat loss (calor) associated with inflammation. Interestingly, diminished production of adenosine leads to dramatic vascular leakage resulting from diminished activation of adenosine A2B receptors on the vascular endothelium (Thompson et al., 2004; Eckle et al., 2008) suggesting that the adenosine released at inflamed sites diminishes the swelling (tumor) that is so prominent at inflamed sites.
Adenosine Inhibits Recruitment and Activation of Neutrophils
Neutrophils are recruited to inflamed sites by a combination of chemokines and adhesive interactions between leukocytes and the vascular endothelium. Adenosine diminishes inflammation by diminishing leukocyte recruitment; adenosine inhibits stimulated neutrophil adhesion to the vascular endothelium (Cronstein et al., 1986) and neutrophil-mediated injury to the endothelium. Adenosine receptor stimulation diminishes neutrophil adhesion to the endothelium by inhibiting both selectin- and integrin-mediated adhesive events (Cronstein et al., 1992; Bullough et al., 1995; Bouma et al., 1996; Sullivan et al., 2004). Presumably these same mechanisms apply to recruitment of other cell types to inflamed sites as well. Although having noted these anti-inflammatory effects of adenosine it has recently been reported that neutrophils release ATP which is converted to adenosine extracellularly and the adenosine binds to A3 receptors to promote chemotaxis and loss or inhibition of A3 receptors markedly reduces leukocyte recruitment to sites of bacterial infection (Chen et al., 2006). Other studies suggest that the A3 receptor-mediated effects on neutrophil recruitment are more selective and may not be important for recruitment to other chemoattractants (Montesinos et al., 2006).
Adenosine diminishes stimulated neutrophil production of oxygen radicals and other potentially deleterious mediators (Reviewed in Taylor et al., 2005). Moreover, adenosine, acting primarily at A2A receptors inhibits phagocytosis of particles (Reviewed in Taylor et al., 2005). Although cAMP mediates many of the downstream effects of adenosine A2A receptors via activation of PKA there have been reports that cAMP-PKA-independent mechanisms mediate inhibition of neutrophil activation by adenosine A2A receptors.
Adenosine and Classical Macrophage Activation
Macrophages are best known for initiating an effective innate immune response against microbes by recognizing pathogen-associated molecular patterns (PAMPs) through pattern-recognition receptors (PRRs) (Pozzi et al., 2005). Following phagocytosis, macrophages destroy most micro-organisms. By producing diverse molecules and presenting antigens to T cells, macrophages in addition to dendritic cells, orient the adaptive immune response leading to the expansion and differentiation of lymphocytes specific for invaders or cancer cells (Gordon and Taylor, 2005; Preynat-Seauve et al., 2006).
Macrophages comprise a heterogeneous population of cells, and show bewildering functional plasticity in response to dynamic micro-environmental cues. Macrophage heterogeneity arises as macrophages differentiate from immature monocyte precursors or yolk-sac macrophages (Mills et al., 2000; Kuroda et al., 2002; Mosser, 2003; Murray and Wynn, 2011). In a conscious parallel with T helper (Th)1 and Th2 lymphocytes, macrophages have been classified into M1 and M2 phenotypes. M1 or “classical” activation of macrophages is induced by toll-like receptor (TLR) agonists, either with or without the Th1 cytokine interferon (IFN)-γ, and results in an inflammatory phenotype characterized by expression of a series of inflammatory cytokines and chemokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1-β, IL-6, IL-12, and macrophage inflammatory protein (MIP)-1α (Mosser and Edwards, 2008; Biswas and Mantovani, 2010). M1 macrophages are strong promoters of Th1 immune responses (Hasko et al., 2000) and have anti-proliferative and cytotoxic activities, which result from their ability to produce reactive oxygen and nitrogen species, such as hydrogen peroxide, superoxide, nitric oxide (NO), and peroxynitrite, and pro-inflammatory cytokines.
The role of adenosine in regulating classical macrophage activation has been studied in detail. As such, adenosine has been shown to be a broad inhibitor of the pro-inflammatory consequences of classical macrophage activation. The anti-inflammatory effects of adenosine on M1 macrophages include suppression of cytokine/chemokine production (Hasko et al., 1996, 1998, 2007; Szabo et al., 1998; Xaus et al., 1999; Sipka et al., 2005, 2007; Ryzhov et al., 2008; Koscso et al., 2012) and NO production (Csoka et al., 2007a; Ryzhov et al., 2008). In contrast to the suppressive effect of adenosine on the production of pro-inflammatory mediators, adenosine augments production of the anti-inflammatory cytokine IL-10 by M1 macrophages. The current consensus is that the regulatory effects of adenosine on M1 macrophages are mediated predominantly by A2A receptors (Hasko et al., 1996, 2009; Pinhal-Enfield et al., 2003; Nemeth et al., 2005; Kreckler et al., 2006; Csoka et al., 2007b; Chen et al., 2009; Wilson et al., 2009; Belikoff et al., 2011). For example, using A2A receptor deficient mice combined with pharmacologic approaches, it has been shown that adenosine inhibits TNF-α, IL-6, and IL-12 release and augments IL-10 production by lipopolysaccharide (LPS)- or bacteria-activated macrophages mostly through A2A receptors (Nemeth et al., 2005; Hasko et al., 2007; Kara et al., 2010a). Although, A2B receptors have been overshadowed by A2A receptors as the primary adenosine receptors shaping the function of M1 macrophages, there is growing evidence that A2B receptors can also become operational in regulating M1 macrophage function. In this context, we recently showed that A2B receptors but not A2A receptors augment LPS-induced IL-10 production by RAW264.7 macrophages, which express high levels of A2B receptors and low levels of A2A receptors (Pinhal-Enfield et al., 2003). Moreover, adenosine can suppress LPS-induced TNF-α production even in A2A receptor deficient mice, and this effect is mediated by A2B receptors (Kara et al., 2010a). A1 adenosine receptors and A3 receptors are expressed at much lower levels on the surface of macrophages and their role in regulating macrophage function remains incompletely understood (Hasko et al., 1996).
Studies utilizing A1 receptor deficient mice recently showed that A1 receptors play a critical role in osteoclast development from monocytic precursors (Merrill et al., 1995). Interestingly, A1 receptors also regulate fusion of human peripheral blood monocytes into giant cells in vitro as well although the mechanism for this regulation has not been fully established (Merrill et al., 1997; Hasko and Pacher, 2012). Genetic studies have yet to confirm the role of A3 receptors in governing macrophage function (Nelms et al., 1999).
Adenosine and Alternative Macrophage Activation
M2 or “alternatively activated” macrophages were originally described as macrophages induced by the Th2 cytokines IL-4 and IL-13. The effects of IL-4 and IL-13 on macrophages partially overlap because they use dimeric receptors that share the IL-4 receptor (IL-4R)α subunit. In contrast, the differences between signaling by IL-4 and IL-13 stem from the fact that while IL-4 is able to activate both the IL-4Rα/common γ chain and IL-4Rα/IL-13Rα dimers, IL-13 can only activate the latter complex. The intracellular signaling pathways are incompletely characterized and involve members of the Janus-activated kinase (JAK) and signal transducer and activator of transcription (STAT) family, especially STAT6. In addition to STAT6 (Gray et al., 2005), recent studies have identified CCAAT-enhancer-binding protein (C/EBP)β (Pauleau et al., 2004; Albina et al., 2005; Ruffell et al., 2009), cAMP response element-binding protein (CREB) (Odegaard et al., 2007), peroxisome proliferator-activated receptor (PPAR)γ (Gallardo-Soler et al., 2008; Satoh et al., 2010; Szanto et al., 2010), IFN regulatory factor (IRF)4 (El Chartouni et al., 2010; Liao et al., 2011), Krüppel-like factor 4 (KLF4) (Takeda et al., 2010), hypoxia-inducible factor-2 (HIF-2) (Pello et al., 2012), and c-MYC (Sica and Mantovani, 2012) as contributors to the transcriptional response driving M2 macrophage activation. Hallmark M2 markers include arginase-1, tissue inhibitor of metalloproteinases (TIMP)-1, macrophage galactose-type C-type lectin (mgl)-1, IL-4Rα, Ym1, and resistin-like molecule (RELM)α (Anthony et al., 2006). Increasingly, activation of multiple markers is used to unequivocally identify M2 macrophages in the context of responses to different antigens (Chen et al., 2012).
M2 macrophages are elicited following infection with multicellular parasites, and can lead to an inflammatory response qualitatively different from and capable of downregulating harmful Th1-type inflammatory responses. Recent studies have suggested that increased M2 macrophage arginase activity during helminth infections is an important element in the control and expulsion of worms (Hesse et al., 2001; Gordon, 2003; Edwards et al., 2006). M2 macrophages contribute to the control of inflammation and can mediate enhanced wound healing through arg-1-mediated production of collagen and insulin-like growth factor 1 (IGF-1), and by contributing to the clearance of cellular debris through scavenger receptors (Gordon, 2003; Anthony et al., 2006). The immunoregulatory/protective, rather than tissue damaging, role of M2 macrophages is also exemplified by the fact that they are abundant in healthy tissues that are associated with naturally immune suppressed states, such as placenta, lung, and other immunologically privileged sites (Noel et al., 2004). In contrast to their protective effects in acute inflammation, it has been proposed that M2 macrophages activated by IL-4 and IL-13 during asthma and chronic obstructive pulmonary disease contribute significantly to airway remodeling and lung fibrosis leading to lung dysfunction (Mantovani et al., 2004; Van Ginderachter et al., 2006; Martinez et al., 2008; Csoka et al., 2012). Additionally, M2 macrophages have also been shown to be hijacked by tumor cells to function as suppressors of anti-tumor T cell responses and stimulators of tumor angiogenesis (Martinez et al., 2008; Koroskenyi et al., 2011). Based on these observations, M2 macrophages have been proposed as an emerging therapeutic target for a variety of disease states.
We have recently discovered that adenosine strongly promotes IL-4/IL-13-induced M2 macrophage activation in vitro, as indicated by upregulation of the arginase-1, TIMP-1, and mgl-1 (Barczyk et al., 2010). Our studies, utilizing both pharmacological approaches and macrophages from adenosine receptor deficient and wild type (WT) mice, indicate that A2B adenosine receptors, and to a lesser degree other adenosine receptors, are required for mediating the stimulatory effect of adenosine on IL-4-induced M2 macrophage activation. Our data also indicate that the stimulatory effect of adenosine receptor activation on M2 macrophage development is mediated by the transcription factor C/EBPβ, but not STAT6 or CREB (Barczyk et al., 2010).
While the designation M2 usually denotes macrophages activated by IL-4 or IL-13, M2 macrophages can also be induced by other anti-inflammatory stimuli, which include immune complexes, and heterogeneous deactivating mediators such as apoptotic cells, glucocorticoids, and IL-10 (Kular et al., 2012). Thus, IL-4/IL-13-activated macrophages are also called M2a, immune complex-activated macrophages are referred to as M2b, and macrophages activated by apoptotic cells, glucocorticoids, and IL-10 are termed M2c. In macrophages phagocytosing apoptotic cells, adenosine released endogenously activates A2A receptors and inhibits the generation of the pro-inflammatory chemokines MIP-2 and cytokine-induced neutrophil-attracting chemokine (KC) (Kara et al., 2010b). Moreover, glucocorticoids promote survival of anti-inflammatory monocytes via upregulation and autocrine activation of A3 adenosine receptors (Mediero et al., 2012a). Together, in macrophages exposed to apoptotic cells and glucocorticoids adenosine switches macrophage phenotype from pro-inflammatory to anti-inflammatory (Nelms et al., 1999).
Adenosine and Myeloid/Monocyte-Derived Syncytial Cells (Osteoclasts and Giant Cells)
Myeloid precursors can, in response to M-CSF and RANKL, differentiate into osteoclasts, multinucleated giant cells that mediate bone resorption (Mediero et al., 2012b). Studies utilizing A1 receptor deficient mice recently showed that A1 receptors play a critical role in osteoclast development from monocytic precursors and bone resorption (Ernst et al., 2010; McNally and Anderson, 2011). In contrast to A1 adenosine receptors, A2A receptors inhibit osteoclast differentiation and function (Deaglio et al., 2007) and A2A receptor stimulation has been shown to inhibit wear particle-induced osteolysis, a form of inflammatory bone destruction resulting from particulates shed from joint prostheses (Chalmin et al., 2012).
Similar to osteoclasts, in response to IFN-γ or other stimuli, monocytes will fuse to form multinucleated giant cells, hallmarks of responses to foreign bodies and such diseases as Sarcoidosis (Semenza, 2010). Interestingly, A1 receptors also regulate fusion of human peripheral blood monocytes into giant cells in vitro as well although the mechanism for this regulation has not been fully established (Merrill et al., 1997; Hasko and Pacher, 2012). Genetic studies have yet to confirm the role of A3 receptors in governing macrophage function (Nelms et al., 1999).
Adenosine and T Cells
Forkhead box P3 (FOXP3)-expressing regulatory T (Treg) cells are crucial in the maintenance of immunological self-tolerance and in the regulation of immune responses. CD39 and CD73 are expressed on the surface of Foxp3+ Tregs and are increasingly used as markers of Tregs (Leibovich et al., 2002). Deaglio et al. (2007) and Adair (2005) showed that CD39 and CD73 on the surface of Tregs produce adenosine, which mediates a substantial portion of the anti-inflammatory and immune regulatory effects of Tregs by engaging A2A receptors on effector T cells. More recently, Th 17 cells were also shown to express CD39 and CD73, which, by producing adenosine, suppress both CD4+ and CD8+ T cell effector functions (Hasko et al., 2009). The expression of both CD39 and CD73 on Th17 cells was upregulated by IL-6 and TGF-β, factors that are crucial for Th17 cell development (Ramanathan et al., 2009).
Adenosine and the Angiogenic Switch in Macrophages
Vascular endothelial growth factor (VEGF) is a potent stimulator of angiogenesis and is crucial for the differentiation of endothelial cells during vasculogenesis, and for the outgrowth of new capillaries from pre-existing blood vessels (Ramanathan et al., 2007). VEGF is thus an important component of tissue repair, and is critical for the resolution of inflammation and wound healing. Macrophages are prominent producers of VEGF during the resolution of inflammation and wound healing. There is a plethora of evidence demonstrating that adenosine promotes angiogenesis, in a large part, by increasing macrophage VEGF production (Murphree et al., 2005; Csoka et al., 2007b; Ernens et al., 2010). It has been shown that adenosine stimulation of A2A receptors on TLR-activated macrophages results in a switch from the production of inflammatory cytokines such as TNF-α and IL-12, to the production of anti-inflammatory and angiogenic factors, including IL-10 and VEGF (Ohta and Sitkovsky, 2001; Gessi et al., 2010) and we termed this process angiogenic switch (Csoka et al., 2007b). This model provides a sequential pathway whereby macrophages initially mediate inflammation through TLR-dependent activation to an M1 phenotype, but are then switched into an angiogenic phenotype by adenosine generated in response to hypoxia/ischemia within the wound area. In addition, the initial activation of macrophages by TLR agonists, which markedly induce expression of adenosine A2A and A2B adenosine receptors, primes these macrophages to respond to increased local levels of extracellular adenosine (Chan and Cronstein, 2010; Gessi et al., 2010). It is noteworthy that while A2A (Csoka et al., 2007b) and A2B (unpublished data) receptors mediate the angiogenic switch in murine macrophages, the adenosine-mediated increase in VEGF secretion by human monocytes is mediated by A2A, A2B, and A3 receptors (Varani et al., 2009, 2011).
Adenosine and Adenosine Receptors in Inflammatory Diseases
From its initial identification as an anti-inflammatory ligand adenosine was thought to be an important endogenous feedback regulator of inflammation and tissue injury. Nonetheless, the first actual demonstration that adenosine, acting at A2A receptors, was an endogenous anti-inflammatory agent had to wait until the development of adenosine receptor knockout mice. Liver injury in response to concanavalin A, a model for viral hepatitis, was markedly enhanced in the absence of adenosine A2A receptors (Khoa et al., 2006), consistent with the hypothesis that, at least in the liver, increased adenosine release at inflamed sites suppresses inflammation and inflammatory injury.
However, making use of adenosine as an anti-inflammatory agent has remained more of a challenge due to the myriad other effects of adenosine acting at A2A and other adenosine receptors, e.g., hypotension. Interestingly, it is now clear that low-dose methotrexate, the anchor drug for the treatment of rheumatoid arthritis, mediates its anti-inflammatory effects via promotion of adenosine release at inflamed sites (Reviewed in Khoa et al., 2001). The “immunosuppressive” effects of methotrexate-mediated inhibition of T cell proliferation are unlikely to account for the effects of methotrexate administered at doses well below those required to inhibit cellular proliferation (15–20 mg/week) and methotrexate is usually accompanied by folic acid or folinic acid supplementation to prevent toxicity without diminishing efficacy. The effects of adenosine, described above, on lymphocyte function provide a better explanation for the immunosuppressive effects of methotrexate, as used to treat Rheumatoid Arthritis (Khoa et al., 2001). Because adenosine receptor expression is increased on leukocytes in patients with Rheumatoid Arthritis (Levy et al., 2006; Hesdorffer et al., 2012), most likely the result of exposure of these cells to high concentrations of TNFα, a cytokine previously demonstrated to increase adenosine A2A receptor expression and function (Smail et al., 1992; Thammavongsa et al., 2009), it is likely that these patients are “primed” to respond to increased adenosine concentrations resulting from methotrexate therapy.
Adenosine and Aging
Immunologic and inflammatory responses are blunted at the beginning and end of life leading to increased susceptibilities to infection for neonates and the elderly. Recent work has suggested that adenosine and its receptors play a role in suppressing responses to infection. Levy et al. (2006) have reported that monocyte/macrophages from neonates are much more sensitive to adenosine A3 receptor-mediated suppression of inflammatory responses (TNF production) than those from adults. In contrast, lymphocytes from the elderly release increased amounts of adenosine leading to suppression of T cell responses (Hesdorffer et al., 2012).
Adenosine is a Virulence Factor Produced by Pathogens
It is not uncommon for invasive pathogens to take advantage of mammalian mechanisms for suppression of host responses to promote spread or survival of the infecting organism. Thus, it was not surprising to find that Candida albicans hyphae release adenosine which suppresses neutrophil-mediated killing of the organism (Smail et al., 1992). More recent studies have demonstrated that Staphylococcus aureus also produce adenosine to avoid killing by the host as well (Thammavongsa et al., 2009). Thus, adenosine, produced by invasive organisms can promote spread of the organism by suppressing host killing of the bacteria.
Conclusion
Adenosine is a potent endogenous anti-inflammatory agent that regulates the function of inflammatory cells via interaction with specific receptors expressed on these cells (Table 1). Already known as an endogenous regulator of inflammation, adenosine also mediates the anti-inflammatory effects of methotrexate, one of the most widely used anti-inflammatory drugs.
Table 1.
Cellular expression of adenosine receptors.
| A1 receptor | A2A receptor | A2B receptor | A3 receptor | |
|---|---|---|---|---|
| Neutrophils | Diminished adhesion, activation | Stimulates chemotaxis | ||
| M1 macrophages | Inhibit phagocytosis, inflammatory cytokine production, increase IL-10 | Stimulates IL-10 | ||
| M2 macrophages | Stimulates M2 macrophage differentiation Production of pro-angiogenic factors |
|||
| Lymphocytes | Inhibits TH17 differentiation Stimulates TReg differentiation |
|||
| Osteoclasts and giant cells | Promotes osteoclast differentiation Stimulates giant cell formation |
Inhibits osteoclast differentiation |
Conflict of Interest Statement
Bruce Cronstein, Intellectual Property: Patents on use of adenosine A2A receptor agonists to promote wound healing and use of A2A receptor antagonists to inhibit fibrosis. Patent on use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone. Patent on use of adenosine A2A agonists to promote bone regeneration. Patent on use of anti-netrin-1 antibodies for the treatment of bone disease. Patent on use of adenosine A2A agonists and A1 antagonists to inhibit wear part.
Acknowledgments
This work was supported by National Institutes of Health Grants R01GM66189 (GH) AR54897 (BNC), AR046121 (BNC), the NYU-HHC Clinical and Translational Science Institute (UL1 TR000038), and LOG#09065004 (Contract W81XWH-10-1-1015) Grant from United States Department of Defense.
References
- Adair T. H. (2005). Growth regulation of the vascular system: an emerging role for adenosine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R283–R296 10.1152/ajpregu.00840.2004 [DOI] [PubMed] [Google Scholar]
- Albina J. E., Mahoney E. J., Daley J. M., Wesche D. E., Morris S. M., Jr., Reichner J. S. (2005). Macrophage arginase regulation by CCAAT/enhancer-binding protein beta. Shock 23, 168–172 10.1097/01.shk.0000148054.74268.e2 [DOI] [PubMed] [Google Scholar]
- Anthony R. M., Urban J. F., Jr., Alem F., Hamed H. A., Rozo C. T., Boucher J. L., et al. (2006). Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12, 955–960 10.1038/nm1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk K., Ehrchen J., Tenbrock K., Ahlmann M., Kneidl J., Viemann D., et al. (2010). Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood 116, 446–455 10.1182/blood-2009-10-247106 [DOI] [PubMed] [Google Scholar]
- Belikoff B. G., Hatfield S., Georgiev P., Ohta A., Lukashev D., Buras J. A., et al. (2011). A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J. Immunol. 186, 2444–2453 10.4049/jimmunol.1001567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S. K., Mantovani A. (2010). Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 10.1038/ni.1937 [DOI] [PubMed] [Google Scholar]
- Bouma M. G., van den Wildenberg F. A., Buurman W. A. (1996). Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am. J. Physiol. 270, C522–C529 [DOI] [PubMed] [Google Scholar]
- Bullough D. A., Magill M. J., Firestein G. S., Mullane K. M. (1995). Adenosine activates A2 receptors to inhibit neutrophil adhesion and injury to isolated cardiac myocytes. J. Immunol. 155, 2579–2586 [PubMed] [Google Scholar]
- Chalmin F., Mignot G., Bruchard M., Chevriaux A., Vegran F., Hichami A., et al. (2012). Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362–373 10.1016/j.immuni.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Chan E. S., Cronstein B. N. (2010). Methotrexate – how does it really work? Nat. Rev. Rheumatol. 6, 175–178 10.1038/nrrheum.2010.5 [DOI] [PubMed] [Google Scholar]
- Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A., et al. (2012). An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266 10.1038/nm.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yang D., Carroll S. H., Eltzschig H. K., Ravid K. (2009). Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp. Hematol. 37, 533–538 10.1016/j.exphem.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., et al. (2006). ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 10.1126/science.1137541 [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. (1986). Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J. Clin. Invest. 78, 760–770 10.1172/JCI112638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Philips M., Hirschhorn R., Abramson S. B., Weissmann G. (1992). Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J. Immunol. 148, 2201–2206 [PubMed] [Google Scholar]
- Csoka B., Nemeth Z. H., Virag L., Gergely P., Leibovich S. J., Pacher P., et al. (2007a). A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 110, 2685–2695 10.1182/blood-2007-01-065870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B., Nemeth Z. H., Selmeczy Z., Koscso B., Pacher P., Vizi E. S., et al. (2007b). Role of A(2A) adenosine receptors in regulation of opsonized E. coli-induced macrophage function. Purinergic Signal. 3, 447–452 10.1007/s11302-007-9075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B., Selmeczy Z., Koscso B., Nemeth Z. H., Pacher P., Murray P. J., et al. (2012). Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26, 376–386 10.1096/fj.11-190934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaglio S., Dwyer K. M., Gao W., Friedman D., Usheva A., Erat A., et al. (2007). Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury A. N., Szent-Gyorgi A. (1929). The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J. Physiol. (Lond.) 68, 213–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T., Faigle M., Grenz A., Laucher S., Thompson L. F., Eltzschig H. K. (2008). A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111, 2024–2035 10.1182/blood-2007-10-117044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. P., Zhang X., Frauwirth K. A., Mosser D. M. (2006). Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307 10.1189/jlb.0406249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Chartouni C., Schwarzfischer L., Rehli M. (2010). Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology 215, 821–825 10.1016/j.imbio.2010.05.032 [DOI] [PubMed] [Google Scholar]
- Ernens I., Leonard F., Vausort M., Rolland-Turner M., Devaux Y., Wagner D. R. (2010). Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem. Biophys. Res. Commun. 392, 351–356 10.1016/j.bbrc.2010.01.023 [DOI] [PubMed] [Google Scholar]
- Ernst P. B., Garrison J. C., Thompson L. F. (2010). Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J. Immunol. 185, 1993–1998 10.4049/jimmunol.1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Soler A., Gomez-Nieto C., Campo M. L., Marathe C., Tontonoz P., Castrillo A., et al. (2008). Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol. Endocrinol. 22, 1394–1402 10.1210/me.2007-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S., Fogli E., Sacchetto V., Merighi S., Varani K., Preti D., et al. (2010). Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler. Thromb. Vasc. Biol. 30, 90–97 10.1161/ATVBAHA.109.194902 [DOI] [PubMed] [Google Scholar]
- Gordon S. (2003). Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- Gordon S., Taylor P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- Gray M. J., Poljakovic M., Kepka-Lenhart D., Morris S. M., Jr. (2005). Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene 353, 98–106 10.1016/j.gene.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Hasko G., Cronstein B. N. (2004). Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 25, 33–39 10.1016/j.it.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Hasko G., Csoka B., Nemeth Z. H., Vizi E. S., Pacher P. (2009). A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 30, 263–270 10.1016/j.it.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Kuhel D. G., Chen J. F., Schwarzschild M. A., Deitch E. A., Mabley J. G., et al. (2000). Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14, 2065–2074 10.1096/fj.99-0508com [DOI] [PubMed] [Google Scholar]
- Hasko G., Linden J., Cronstein B., Pacher P. (2008). Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Nemeth Z. H., Vizi E. S., Salzman A. L., Szabo C. (1998). An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur. J. Pharmacol. 358, 261–268 10.1016/S0014-2999(98)00619-0 [DOI] [PubMed] [Google Scholar]
- Hasko G., Pacher P. (2012). Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 32, 865–869 10.1161/ATVBAHA.111.226852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Pacher P., Deitch E. A., Vizi E. S. (2007). Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol. Ther. 113, 264–275 10.1016/j.pharmthera.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Pacher P., Vizi E. S., Illes P. (2005). Adenosine receptor signaling in the brain immune system. Trends Pharmacol. Sci. 26, 511–516 10.1016/j.tips.2005.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G., Szabo C., Nemeth Z. H., Kvetan V., Pastores S. M., Vizi E. S. (1996). Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 157, 4634–4640 [PubMed] [Google Scholar]
- Hesdorffer C. S., Malchinkhuu E., Biragyn A., Mabrouk O. S., Kennedy R. T., Madara K., et al. (2012). Distinctive immunoregulatory effects of adenosine on T cells of older humans. FASEB J. 26, 1301–1310 10.1096/fj.11-197046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Modolell M., La Flamme A. C., Schito M., Fuentes J. M., Cheever A. W., et al. (2001). Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 [DOI] [PubMed] [Google Scholar]
- Kara F. M., Chitu V., Sloane J., Axelrod M., Fredholm B. B., Stanley E. R., et al. (2010a). Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 24, 2325–2333 10.1096/fj.09-147447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara F. M., Doty S. B., Boskey A., Goldring S., Zaidi M., Fredholm B. B., et al. (2010b). Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum. 62, 534–541 10.1002/art.27219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa N. D., Montesinos M. C., Reiss A. B., Delano D., Awadallah N., Cronstein B. N. (2001). Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J. Immunol. 167, 4026–4032 [DOI] [PubMed] [Google Scholar]
- Khoa N. D., Postow M., Danielsson J., Cronstein B. N. (2006). Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol. Pharmacol. 69, 1311–1319 10.1124/mol.105.016857 [DOI] [PubMed] [Google Scholar]
- Koroskenyi K., Duro E., Pallai A., Sarang Z., Kloor D., Ucker D. S., et al. (2011). Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. J. Immunol. 186, 7144–7155 10.4049/jimmunol.1002284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscso B., Csoka B., Selmeczy Z., Himer L., Pacher P., Virag L., et al. (2012). Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J. Immunol. 188, 445–453 10.4049/jimmunol.1101224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler L. M., Wan T. C., Ge Z. D., Auchampach J. A. (2006). Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J. Pharmacol. Exp. Ther. 317, 172–180 10.1124/jpet.105.096016 [DOI] [PubMed] [Google Scholar]
- Kular J., Tickner J., Chim S. M., Xu J. (2012). An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 45, 863–873 10.1016/j.clinbiochem.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Kuroda E., Kito T., Yamashita U. (2002). Reduced expression of STAT4 and IFN-gamma in macrophages from BALB/c mice. J. Immunol. 168, 5477–5482 [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Chen J. F., Pinhal-Enfield G., Belem P. C., Elson G., Rosania A., et al. (2002). Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am. J. Pathol. 160, 2231–2244 10.1016/S0002-9440(10)61170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Coughlin M., Cronstein B. N., Roy R. M., Desai A., Wessels M. R. (2006). The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 177, 1956–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Sharma N., Kapadia F., Zhou G., Lu Y., Hong H., et al. (2011). Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 10.1172/JCI45444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. (2001). Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41, 775–787 10.1146/annurev.pharmtox.41.1.775 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- Martinez F. O., Sica A., Mantovani A., Locati M. (2008). Macrophage activation and polarization. Front. Biosci. 13, 453–461 10.2741/2692 [DOI] [PubMed] [Google Scholar]
- McNally A. K., Anderson J. M. (2011). Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 713, 97–111 10.1007/978-94-007-0763-4_7 [DOI] [PubMed] [Google Scholar]
- Mediero A., Kara F. M., Wilder T., Cronstein B. N. (2012a). Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am. J. Pathol. 180, 775–786 10.1016/j.ajpath.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediero A., Frenkel S. R., Wilder T., He W., Mazumder A., Cronstein B. N. (2012b). Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci. Transl. Med. 4, 135ra65. 10.1126/scitranslmed.3003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. T., Coffey D., Shen C., Zakharenko O., Zhang H. W., Lahita R. G., et al. (1995). Mechanisms of rheumatoid nodulosis: methotrexate-enhanced monocyte fusion requires protein synthesis and intact microtubules. Arthritis Rheum. 38(Suppl.), S157. 10.1002/art.1780381118 [DOI] [Google Scholar]
- Merrill J. T., Cronstein B. N., Mitnick H., Goodman S., Diakolios C., Paget S., et al. (1997). Reversal of new but not old rheumatoid nodules by colchicine: evidence from an in vitro model and case reports of 14 patients. J. Clin. Rheumatol. 3, 328–333 10.1097/00124743-199712000-00005 [DOI] [PubMed] [Google Scholar]
- Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. (2000). M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–617310843666 [Google Scholar]
- Montesinos M. C., Desai A., Cronstein B. N. (2006). Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res. Ther. 8, R53. 10.1186/ar1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M. (2003). The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212 10.1189/jlb.0602325 [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edwards J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree L. J., Sullivan G. W., Marshall M. A., Linden J. (2005). Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem. J. 391, 575–580 10.1042/BJ20050888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J., Wynn T. A. (2011). Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K., Keegan A. D., Zamorano J., Ryan J. J., Paul W. E. (1999). The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17, 701–738 10.1146/annurev.immunol.17.1.701 [DOI] [PubMed] [Google Scholar]
- Nemeth Z. H., Lutz C. S., Csoka B., Deitch E. A., Leibovich S. J., Gause W. C., et al. (2005). Adenosine Augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J. Immunol. 175, 8260–8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel W., Raes G., Hassanzadeh Ghassabeh G., De Baetselier P., Beschin A. (2004). Alternatively activated macrophages during parasite infections. Trends Parasitol. 20, 126–133 10.1016/j.pt.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., et al. (2007). Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Sitkovsky M. (2001). Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 10.1038/414916a [DOI] [PubMed] [Google Scholar]
- Pauleau A. L., Rutschman R., Lang R., Pernis A., Watowich S. S., Murray P. J. (2004). Enhancer-mediated control of macrophage-specific arginase I expression. J. Immunol. 172, 7565–7573 [DOI] [PubMed] [Google Scholar]
- Pello O. M., De Pizzol M., Mirolo M., Soucek L., Zammataro L., Amabile A., et al. (2012). Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 119, 411–421 10.1182/blood-2011-02-339911 [DOI] [PubMed] [Google Scholar]
- Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S. N., Salzman A. L., Boons G. J., et al. (2003). An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 163, 711–721 10.1016/S0002-9440(10)63698-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L. A., Maciaszek J. W., Rock K. L. (2005). Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J. Immunol. 175, 2071–2081 [DOI] [PubMed] [Google Scholar]
- Preynat-Seauve O., Schuler P., Contassot E., Beermann F., Huard B., French L. E. (2006). Tumor-infiltrating dendritic cells are potent antigen-presenting cells able to activate T cells and mediate tumor rejection. J. Immunol. 176, 61–67 [DOI] [PubMed] [Google Scholar]
- Ramanathan M., Luo W., Csoka B., Hasko G., Lukashev D., Sitkovsky M. V., et al. (2009). Differential regulation of HIF-1alpha isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists. J. Leukoc. Biol. 86, 681–689 10.1189/jlb.0109021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Pinhal-Enfield G., Hao I., Leibovich S. J. (2007). Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol. Biol. Cell 18, 14–23 10.1091/mbc.E06-07-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell D., Mourkioti F., Gambardella A., Kirstetter P., Lopez R. G., Rosenthal N., et al. (2009). A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. U.S.A. 106, 17475–17480 10.1073/pnas.0908641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S., Zaynagetdinov R., Goldstein A. E., Novitskiy S. V., Blackburn M. R., Biaggioni I., et al. (2008). Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J. Pharmacol. Exp. Ther. 324, 694–700 10.1124/jpet.107.131540 [DOI] [PubMed] [Google Scholar]
- Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y., et al. (2010). The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 10.1038/ni.1920 [DOI] [PubMed] [Google Scholar]
- Semenza G. L. (2010). Vascular responses to hypoxia and ischemia. Arterioscler. Thromb. Vasc. Biol. 30, 648–652 10.1161/ATVBAHA.108.181644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipka S., Kovacs I., Szanto S., Szegedi G., Brugos L., Bruckner G., et al. (2005). Adenosine inhibits the release of interleukin-1beta in activated human peripheral mononuclear cells. Cytokine 31, 258–263 10.1016/j.cyto.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Sipka S., Kovacs I., Szanto S., Szegedi G., Brugos L., Bruckner G., et al. (2007). Adenosine inhibits the release of arachidonic acid and its metabolites (AAM) in activated human peripheral mononuclear cells. Inflamm. Res. 56, 468–472 10.1007/s00011-007-7102-6 [DOI] [PubMed] [Google Scholar]
- Sitkovsky M. V., Lukashev D., Apasov S., Kojima H., Koshiba M., Caldwell C., et al. (2004). Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 10.1146/annurev.immunol.22.012703.104731 [DOI] [PubMed] [Google Scholar]
- Smail E. H., Cronstein B. N., Meshulam T., Esposito A. L., Ruggeri R. W., Diamond R. D. (1992). In vitro, Candida albicans releases the immune modulator adenosine and a second, high-molecular weight agent that blocks neutrophil killing. J. Immunol. 148, 3588–3595 [PubMed] [Google Scholar]
- Sullivan G. W., Lee D. D., Ross W. G., DiVietro J. A., Lappas C. M., Lawrence M. B., et al. (2004). Activation of A2A adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. J. Leukoc. Biol. 75, 127–134 10.1189/jlb.0603300 [DOI] [PubMed] [Google Scholar]
- Szabo C., Scott G. S., Virag L., Egnaczyk G., Salzman A. L., Shanley T. P., et al. (1998). Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br. J. Pharmacol. 125, 379–387 10.1038/sj.bjp.0702040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto A., Balint B. L., Nagy Z. S., Barta E., Dezso B., Pap A., et al. (2010). STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 33, 699–712 10.1016/j.immuni.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., O’Dea E. L., Doedens A., Kim J. W., Weidemann A., Stockmann C., et al. (2010). Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24, 491–501 10.1101/gad.1881410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. R., Martinez-Pomares L., Stacey M., Lin H. H., Brown G. D., Gordon S. (2005). Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 10.1146/annurev.immunol.23.021704.115816 [DOI] [PubMed] [Google Scholar]
- Thammavongsa V., Kern J. W., Missiakas D. M., Schneewind O. (2009). Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 206, 2417–2427 10.1084/jem.20090097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. F., Eltzschig H. K., Ibla J. C., Van De Wiele C. J., Resta R., Morote-Garcia J. C., et al. (2004). Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395–1405 10.1084/jem.20040915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginderachter J. A., Movahedi K., Hassanzadeh Ghassabeh G., Meerschaut S., Beschin A., Raes G., et al. (2006). Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211, 487–501 10.1016/j.imbio.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Varani K., Massara A., Vincenzi F., Tosi A., Padovan M., Trotta F., et al. (2009). Normalization of A2A and A3 adenosine receptor up-regulation in rheumatoid arthritis patients by treatment with anti-tumor necrosis factor alpha but not methotrexate. Arthritis Rheum. 60, 2880–2891 10.1002/art.24487 [DOI] [PubMed] [Google Scholar]
- Varani K., Padovan M., Vincenzi F., Targa M., Trotta F., Govoni M., et al. (2011). A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res. Ther. 13, R197. 10.1186/ar3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Ross W. G., Agbai O. N., Frazier R., Figler R. A., Rieger J., et al. (2009). The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 182, 4616–4623 10.4049/jimmunol.0801279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaus J., Mirabet M., Lloberas J., Soler C., Lluis C., Franco R., et al. (1999). IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J. Immunol. 162, 3607–3614 [PubMed] [Google Scholar]
- Yegutkin G. G. (2008). Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 10.1016/j.bbamcr.2008.01.024 [DOI] [PubMed] [Google Scholar]