Abstract
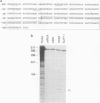
The SIL (SCL interrupting locus) gene was initially discovered at the site of a genomic rearrangement in a T-cell acute lymphoblastic leukemia cell line. This rearrangement, which occurs in a remarkably site-specific fashion, is present in the leukemic cells of 16 to 26% of patients with T-cell acute lymphoblastic leukemia. We have now cloned a normal SIL cDNA from a cell line which does not carry the rearrangement. The SIL cDNA has a long open reading frame of 1,287 amino acids, with a predicted molecular size of 143 kDa. The predicted protein is not homologous with any previously described protein; however, a potential eukaryotic topoisomerase I active site was identified. Cross-species hybridization using a SIL cDNA probe indicated that the SIL gene was conserved in mammals. A survey of human and murine cell lines and tissues demonstrated SIL mRNA to be ubiquitously expressed, at low levels, in hematopoietic cell lines and tissues. With the exception of 11.5-day-old mouse embryos, SIL mRNA was not detected in nonhematopoietic tissues. The genomic structure of SIL was also analyzed. The gene consists of 18 exons distributed over 70 kb, with the 5' portion of the gene demonstrating alternate exon utilization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Flowers A., Davis B. J. Direct implantation and serial transplantation of human acute lymphoblastic leukemia in hamsters, SB-2. Cancer Res. 1968 Jun;28(6):1121–1125. [PubMed] [Google Scholar]
- Aguilera A., Klein H. L. HPR1, a novel yeast gene that prevents intrachromosomal excision recombination, shows carboxy-terminal homology to the Saccharomyces cerevisiae TOP1 gene. Mol Cell Biol. 1990 Apr;10(4):1439–1451. doi: 10.1128/mcb.10.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan P. D., Begley C. G., Bertness V., Nussmeier M., Ezquerra A., Coligan J., Kirsch I. R. The SCL gene is formed from a transcriptionally complex locus. Mol Cell Biol. 1990 Dec;10(12):6426–6435. doi: 10.1128/mcb.10.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Barber T. X. Dream Deprivation. Science. 1960 Nov 11;132(3437):1417–1418. doi: 10.1126/science.132.3437.1417. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Davey M. P., Nakahara K., Tchorz K., Kurtzberg J., Hershfield M. S., Haynes B. F., Cohen D. I., Waldmann T. A. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2031–2035. doi: 10.1073/pnas.86.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Eng W. K., Pandit S. D., Sternglanz R. Mapping of the active site tyrosine of eukaryotic DNA topoisomerase I. J Biol Chem. 1989 Aug 15;264(23):13373–13376. [PubMed] [Google Scholar]
- Finger L. R., Kagan J., Christopher G., Kurtzberg J., Hershfield M. S., Nowell P. C., Croce C. M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht F., Morgan R., Hecht B. K., Smith S. D. Common region on chromosome 14 in T-cell leukemia and lymphoma. Science. 1984 Dec 21;226(4681):1445–1447. doi: 10.1126/science.6438800. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Tilton J., Peterson W. D., Jr Identification of T cell lymphoma tumor antigens on human T cell lines. Am J Hematol. 1976;1(2):219–223. doi: 10.1002/ajh.2830010206. [DOI] [PubMed] [Google Scholar]
- Kirsch I. R., Brown J. A., Lawrence J., Korsmeyer S. J., Morton C. C. Translocations that highlight chromosomal regions of differentiated activity. Cancer Genet Cytogenet. 1985 Oct;18(2):159–171. doi: 10.1016/0165-4608(85)90066-4. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Lynn R. M., Bjornsti M. A., Caron P. R., Wang J. C. Peptide sequencing and site-directed mutagenesis identify tyrosine-727 as the active site tyrosine of Saccharomyces cerevisiae DNA topoisomerase I. Proc Natl Acad Sci U S A. 1989 May;86(10):3559–3563. doi: 10.1073/pnas.86.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Caron P. R., Kim R. A. The role of DNA topoisomerases in recombination and genome stability: a double-edged sword? Cell. 1990 Aug 10;62(3):403–406. doi: 10.1016/0092-8674(90)90002-v. [DOI] [PubMed] [Google Scholar]