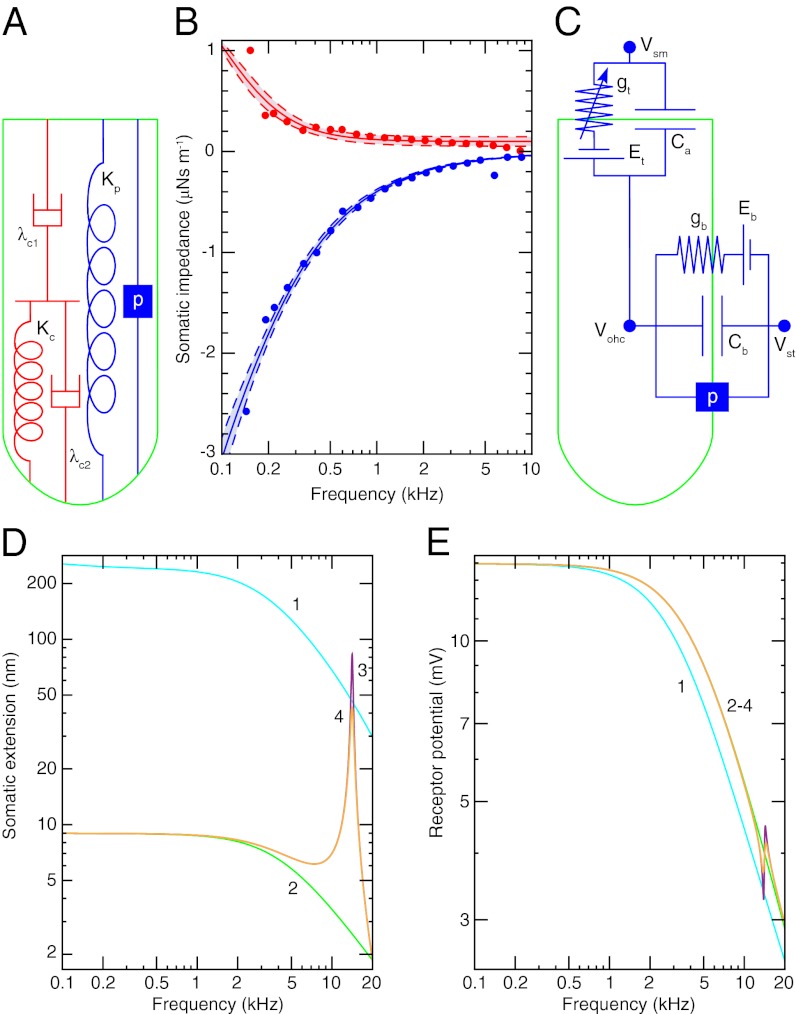
Fig. 2.
(A) The cortical lattice and subsurface cisternae of an OHC are described by the two dashpots λc1 and λc2 and the spring Kc. The plasma membrane is represented by a spring Kp in parallel with a piezoelectric element p betokening the prestin molecules. (B) The experimentally determined real part (red circles) and imaginary part (blue circles) of the mechanical impedance of a 14-kHz OHC are simultaneously fit by the model depicted in A with the constraints of no electromotility, 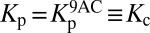 , and p = 0 (R2 > 0.98, solid lines). The fit yields Kc = 1.32 ± 0.04 mN⋅m−1 (P value < 10−28), λc1 = 2.00 ± 0.28 μN⋅s⋅m−1 (P value < 10−7), and λc2 = 104 ± 25 nN⋅s⋅m−1 (P value < 0.001). The dashed lines represent 95% confidence intervals. (C) The transduction current flowing across the apical membrane of the OHC responds to changes in the potential difference between the hair cell’s interior and the scala media, Vohc − Vsm. The potentials of the scala media Vsm at the OHC’s apical surface and of the scala tympani Vst at the OHC’s basolateral membrane are approximately constant. The apical membrane acts as a capacitor Ca in parallel with a variable conductance gt that represents the transduction channels and has a reversal potential Et. Driven by the reversal potential of the basolateral somatic membrane Eb, current exits the OHC through its basolateral membrane, which is described by a conductance gb in parallel with a capacitor Cb and a piezoelectric element p. (D) The model predicts the change in somatic length of a 14-kHz OHC in response to sinusoidal changes in hair-bundle conductance of maximal amplitude around the reference value (SI Appendix, Table S1). Four conditions are considered: (1) an isolated OHC, (2) an OHC loaded by the stiffness Kbm of the basilar membrane, (3) the same after the addition of the mass mbm of the basilar membrane, and (4) the same after the inclusion of the damping λbm of the basilar membrane. (E) The receptor potential is shown under the identical circumstances.
, and p = 0 (R2 > 0.98, solid lines). The fit yields Kc = 1.32 ± 0.04 mN⋅m−1 (P value < 10−28), λc1 = 2.00 ± 0.28 μN⋅s⋅m−1 (P value < 10−7), and λc2 = 104 ± 25 nN⋅s⋅m−1 (P value < 0.001). The dashed lines represent 95% confidence intervals. (C) The transduction current flowing across the apical membrane of the OHC responds to changes in the potential difference between the hair cell’s interior and the scala media, Vohc − Vsm. The potentials of the scala media Vsm at the OHC’s apical surface and of the scala tympani Vst at the OHC’s basolateral membrane are approximately constant. The apical membrane acts as a capacitor Ca in parallel with a variable conductance gt that represents the transduction channels and has a reversal potential Et. Driven by the reversal potential of the basolateral somatic membrane Eb, current exits the OHC through its basolateral membrane, which is described by a conductance gb in parallel with a capacitor Cb and a piezoelectric element p. (D) The model predicts the change in somatic length of a 14-kHz OHC in response to sinusoidal changes in hair-bundle conductance of maximal amplitude around the reference value (SI Appendix, Table S1). Four conditions are considered: (1) an isolated OHC, (2) an OHC loaded by the stiffness Kbm of the basilar membrane, (3) the same after the addition of the mass mbm of the basilar membrane, and (4) the same after the inclusion of the damping λbm of the basilar membrane. (E) The receptor potential is shown under the identical circumstances.