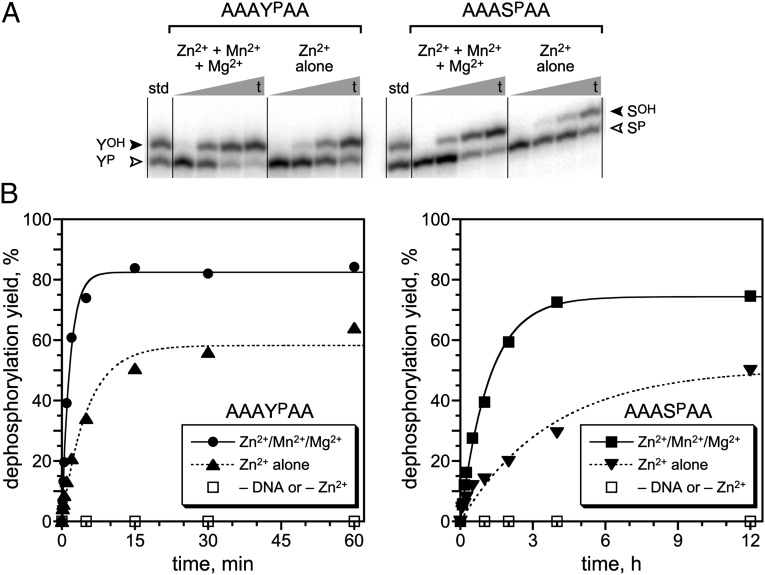
Fig. 2.
Single-turnover assays of the 14WM9 deoxyribozyme that dephosphorylates phosphotyrosine and phosphoserine side chains. (A) PAGE assays of the 14WM9-catalyzed reactions, using DNA-anchored hexapeptide substrates and showing representative time points. The open arrowhead marks the phosphopeptide substrate; the filled arrowhead marks the dephosphorylated product. Each hexapeptide substrate was joined via its N-terminal Ala residue to the 3′-end of the DNA anchor by reductive amination, as described in SI Materials and Methods. Assay conditions: 1 µM 14WM9, 12.5 nM 5′-32P-radiolabeled DNA-anchored hexapeptide, 70 mM Hepes (pH 7.5), 1 mM ZnCl2, 20 mM MnCl2, 40 mM MgCl2, and 150 mM NaCl at 37 °C. In the assays with Zn2+ alone, the Mn2+ and Mg2+ were omitted. Time points for YP substrate: 10 s, 1 min, 5 min, 1 h. Time points for SP substrate: 30 s, 15 min, 2 h, 12 h. (B) Kinetic plots for the assays. The negative controls without deoxyribozyme or without Zn2+ all showed no detectable dephosphorylation activity (<0.5%).