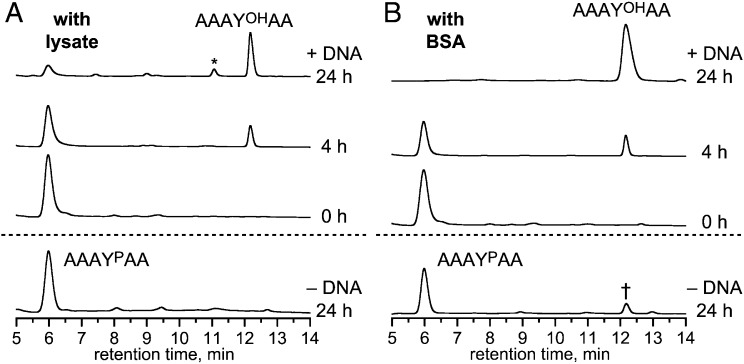
Fig. 5.
Activity of the 14WM9 deoxyribozyme in the presence of human cell lysate and BSA. (A) HPLC multiple-turnover assay with free AAAYPAA peptide in the presence of human cell lysate (160 µg/mL of lysate protein), under conditions of Fig. 3A. The asterisk denotes the pentapeptide AAYOHAA formed by nonspecific peptidase activity in the lysate, as validated by electrospray ionization MS ([M+H]+ m/z calculated 465.2, found 465.2). (B) HPLC multiple-turnover assay in the presence of 20 mg/mL BSA, under conditions of Fig. 3A except 10 mM Zn2+. Substantial activity was also observed at 6 and 8 mM Zn2+, but reduced presumably due to nonspecific chelation of Zn2+ by BSA (Fig. S10). The dagger denotes a small amount (∼14%) of dephosphorylation product formed in 24 h in the absence of 14WM9. In both panels, omission of Zn2+ rather than 14WM9 led to no activity in 24 h. This finding suggests for B that Zn2+ activates nonspecific phosphatases present in the BSA, and inclusion of the DNA suppresses this phosphatase activity.