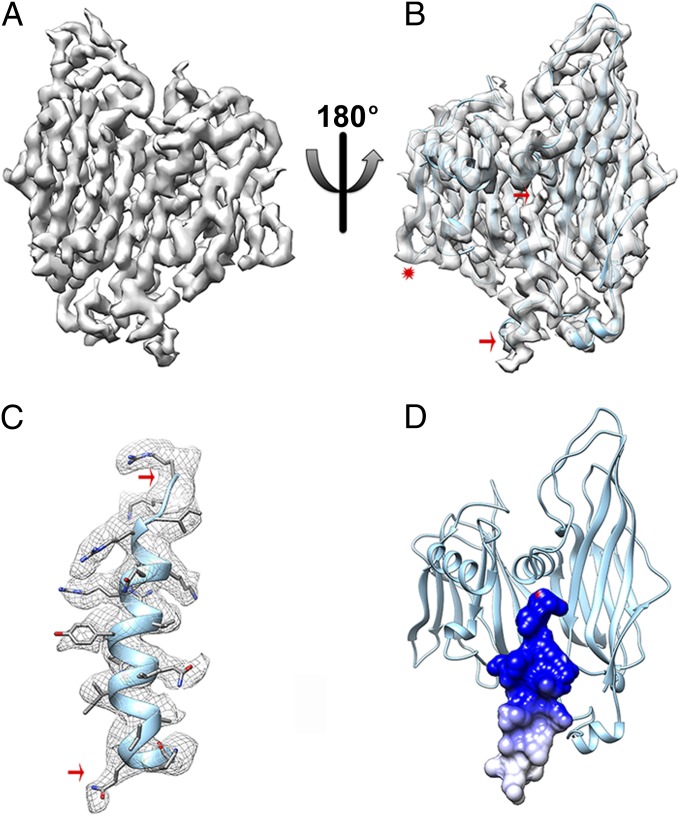
Fig. 2.
The capsid shell. (A) CryoEM density of a single B345 coat subunit at 3.9 Å resolution. (B) Fit of the B345 crystal structure into the corresponding density. The crystal structure stops at residue 324 whereas the cryoEM density is continuous until the C-terminal residue and folds as an α-helix (delineated by the two red arrows). The red star indicates the position of the E-F loop belonging to the smaller jelly-roll of the B345 subunit. (C) The B345 C-terminal α-helix (residues 325–345) was modeled de novo in the cryoEM density. The red arrows are matching the region shown in B. (D) The electrostatic surface potential of the B345 C-terminal helix reveals that its most C-terminal moiety is strongly positively charged due to the presence of many basic residues. Electrostatics calculations were carried out at pH 3.0 and 80 °C, and the result is displayed colored from red (−5 kT/e) to blue (+5 kT/e).