Fig. 1.
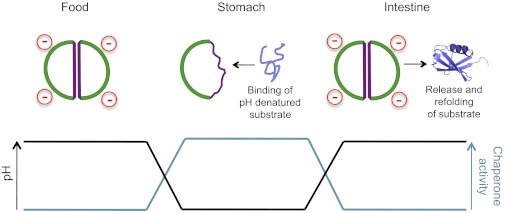
pH-dependent activity of HdeA (in green and purple outline) allows it to have maximal chaperone activity in the low-pH environment of the stomach. Here, periplasmic proteins (example in blue) will be destabilized by the harsh acidic conditions. Binding to HdeA protects them such that they may be released for refolding upon arrival of the bacterium in the small intestine. The work of Foit et al. (2), reported in PNAS, reveals how protonation of two key aspartic acid residues shifts HdeA to a partially unfolded, chaperone-active monomeric state.
