Abstract
Sleep disorders are a common and poorly treated disease state. This double blind, four arm placebo-controlled, randomized trial compared (1) low dose trazodone, (2) Sentra PM, a neurotransmitter based medical food, (3) the joint administration of trazodone and the medical food Sentra PM and (4) placebo. There were 111 subjects studied in 12 independent sites. Subjects underwent baseline screening, informed consent and an initial sleep questionnaire. After 14 days subjects underwent a second evaluation by questionnaire. At baseline and Day 14 the subjects underwent 24 hour ECG recordings that were analyzed in the frequency domain of heart rate variability. The specific high frequency parasympathetic autonomic nervous system activity was analyzed. The primary endpoints were sleep latency and parasympathetic autonomic nervous system improvement in sleeping hours. The results showed improvement in sleep latency for the Sentra PM and combination of Sentra PM and trazodone (−41 and −56 minutes P < 0.001). There was an improvement in quality of sleep for the amino acid formulation Sentra PM and the combination (3.86 and 6.48 Likert units on a 10 point scale P < 0.001). There was an activation of circadian activity percent at night in the medical food and combination groups while there was no change in parasympathetic activity in either the placebo or trazodone group. These data indicate that Sentra PM can improve the quality of sleep, the response to trazodone as a sleep medication and parasympathetic autonomic nervous system activity.
Keywords: medical food, sleep disorder, autonomic nervous system, parasympathetic nervous system, amino acids, heart rate variability, Sentra PM, trazodone, depression, anxiety
Introduction
Restorative sleep is essential for a variety of central nervous system functions including mood, memory and cognition.1,2 Sleep impacts feelings of depression and perception of pain.3–12 Circadian rhythms initiate and induce the stages of sleep as well as promote wakefulness. Sleep is controlled by a series of neurotransmitters including serotonin and acetylcholine which are produced from their amino acid precursors tryptophan and choline. Cycling neurotransmitters determine the sleep stages including phase IV delta and REM sleep.13–19 Adequate production and timely release of these neurotransmitters are necessary for the production of sleep cycles.
Many sleep inducing agents interfere with the reuptake of neurotransmitters, leading to an increase of neuron concentration in the cleft with concurrent intracellular depletion of neurotransmitters including serotonin and acetylcholine. Side effects of sleep medications include depression, daytime somnolence and memory impairment. They may also reduce or abolish REM sleep. Trazodone was chosen for this study as it is the most commonly prescribed sleep aid in the United States even though the primary indication is for depression.
The stages of sleep and circadian autonomic nervous system function can be evaluated using high resolution 24 hour ECG analysis. Parasympathetic autonomic nervous system function is assessed by focusing on the high frequency (HF) band of the heart rate variability signal. In normal subjects, parasympathetic activation and slowing of the heart rate occurs at the initiation of sleep and increases prior to awakening. The activation of the parasympathetic system during sleep should slow the heart rate which becomes more rapid with REM activity. This methodology objectively assesses changes in sleep cycles. The 24 hour ECG recording, in either the time or frequency domain, is established method for assessing autonomic nervous system activity.20–27 In the time domain, two measurements are most commonly used. Total HRV is measured by SDNN, parasympathetic activity by the RMSSD and sympathetic activity by the fill in. In the frequency domain, sympathetic activity is measured by the low frequency band. The high frequency band is a pure parasympathetic autonomic nervous system measurement with no overlap to other sympathetic influences.23,24,28–35
We recently described nutritional modification of autonomic activity by providing amino acid precursors and other agents to trigger timed release of neurotransmitters associated with sleep cycles and pain syndromes. Our group demonstrated that co-administration of neurotransmitter precursors with a pharmaceutical drug can preserve or enhance the efficacy of a medication at the lowest FDA approved dose.36,37
Medical foods49 are a distinct FDA regulatory category different from single molecule chemical pharmaceuticals and dietary supplements. The FDA has regulated amino acid preparations as drugs since the 1940s because they can elicit pharmacologic effects similar to conventional single molecule pharmaceuticals. The best known amino acid preparations are used to treat conditions such as maple syrup disease. An official definition and categorization of medical foods was made in 1988 as part of the Orphan Drug Act. Medical foods are regulated similarly to drugs except they do not require preapproval because their ingredients are generally recognized as safe (GRAS) and their claims are confined to the nutritional management of a specific disease. Medical food claims must be supported by recognized scientific data as determined by medical evaluation. There has been growth in the number of medical foods and in their clinical applications.
The medical food used in this study contains precursors to serotonin and acetylcholine in a patented system that promotes amino acid uptake and neurotransmitter release. The concentrations of amino acids are provided in low milligram doses. Sentra PM promotes specific neurotransmitter production. The amino acid precursors in the formulation augment neurotransmitters proven to be deficient in patients with sleep disorders. Serotonin and acetylcholine initiate sleep, elicit REM sleep and promote delta sleep.38–43 Serotonergic activity increases during wakefulness and is necessary to induce sleep and serotonin deficiencies that lead to insomnia. Serotonin is also involved in wakefulness. Acetylcholine activity is crucial in promoting REM sleep and agonist or supplementation leads to increased REM sleep. The precursor 5-hydroxytryptophan is converted to serotonin to initiate sleep; choline is converted to acetylcholine, bursts of which are essential for REM sleep. The preparation is a patented five component system to (1) provide a neurotransmitter precursor, (2) stimulate uptake of the amino acids before deamination, (3) trigger neurotransmitter release, (4) a system to relieve the adenosine brake which slows neurotransmitter synthesis and release, (5) polyphenols to prevent receptor up regulation. The five part system allows for a substantial reduction of the neurotransmitter precursors used in the amino acid preparation and prevents attenuation.44
Materials and Methods
This four arm, double blind placebo controlled trial of trazodone 50 mg at bedtime, to the amino acid preparation Sentra PM, the co administration of the two agents, and placebo involved 111 subjects. We measured sleep parameters, assessed depression, anxiety and performed 24 hour ECG analysis for HRV.
Prior to site selection, the study was approved by an independent review board. Each subject was administered and signed an informed consent document at each site prior to beginning any study activities.
Protocol
The study was conducted at twelve independent sites around the United States. At each site informed consent was obtained, screening procedures were performed including complete blood count (CBC), comprehensive metabolic panel, height, weight, and blood pressure. The metabolic panel and CBC were monitored to assess possible hepatic, renal, or gastrointestinal toxicity due to study drugs.
Subjects were identified in response to solicitation of persons interested in being prescribed a medical food that may support restful sleep. Subjects were enrolled in 12 separate physicians’ offices. Men and non-pregnant, non-lactating women between the ages of 18 and 75 years with a history of sleep disturbance lasting more than six weeks and defined by perceived lack of restorative sleep were enrolled by the study physician in each site. Subjects currently taking tri-cyclic anti-depressants were excluded, as well as subjects who had previously taken Sentra PM, trazodone or another amino acid formulation. Subjects with biochemical abnormalities that would put the subject at risk or invalidate study findings were excluded. Lactating or pregnant females and subjects with pacemakers or other implanted electrical devices were excluded.
The study involved 111 subjects in a four arm double blind randomized trial comparing placebo alone (N = 25), trazodone alone (N = 36), Sentra PM alone (N = 28), or the combined use of Sentra PM and trazodone (N = 22). On the Day 1 visit, the subjects were randomized to one of four groups: (1) trazodone alone group which was treated with a two capsule dose of a Sentra PM-like placebo at bedtime and trazodone 50 mg daily at bed time (2) Sentra PM alone group which was treated with the active Sentra PM at a two capsule dose at bedtime and a single trazodone like placebo at bedtime, (3) the combined group which were treated with active Sentra PM at a two capsule dose at bedtime and an active trazodone 50 mg at bedtime, and (4) a placebo dose of two Sentra PM placebo and trazodone placebo at bedtime. The active and trazodone tablets were identical. The Sentra PM active and placebo capsules were identical. The study was approved by an independent review board prior to subject enrollment.
A 24 hour ECG recording was performed for heart rate variability (HRV) analysis of autonomic nervous system function.45 After the washout period, there was a baseline Day 1 visit when the Pittsburgh Sleep Quality Index (PSQI) and a Leeds Sleep Evaluation Visual Analogue Scale (LSEQ) were performed.46 The subjects filled out PSQI and LSEQ forms daily for the next 14 days. On Day 14 the subjects returned for repeat blood sampling and a final 24 hour ECG recording.
Primary endpoint
The primary endpoint of the study was quality of sleep as assessed by sleep latency and morning grogginess measured by the LSEQ and the PSQI.47,48 Secondary endpoints included duration of sleep, number of awakenings, snoring, depression and anxiety scores and 24 hour monitoring of HRV.
Safety
There were no adverse events or complications reported among any of the groups during the duration of the study.
24-Hour ECG
The high frequency (HF) band of the frequency transformation of the QRS interval reflects parasympathetic autonomic nervous system activity. From midnight to 5 am circadian rhythms showed increased parasympathetic activity. Sleep disorders are associated with failure to activate this surge in parasympathetic activity.
In this analysis, the HF band was measured at midnight as baseline. We then measured 5 minute epochs at 15 minute intervals until 5 am with 20 epochs measured. Each of these epochs was compared to the midnight baseline for each subject. This was expressed as a percent change for each epoch. The sum of the 20 epochs was computed for each subject at baseline and at day 14. The sum of the 20 epochs has been termed the circadian index. Thus, for each of the 111 subjects, we analyzed approximately 17,000 beats estimated at a heart rate of 80 for these measurements. A total of 1,860,000 beats were analyzed for this analysis. The number of beats of each epoch as a sum at baseline was compared to the same measurement at day 14. The data of the delta was statistically compared.
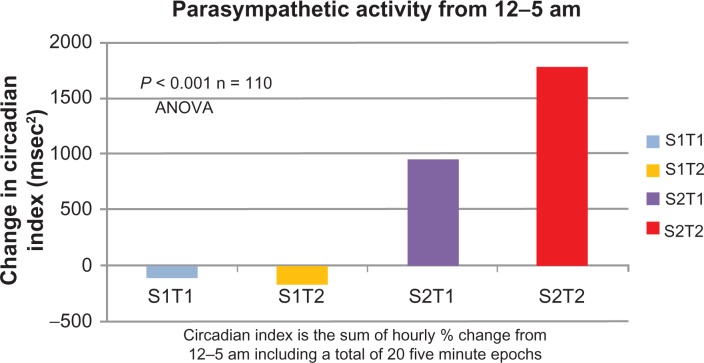
The results are outlined in Table 1 and Figure 1. The placebo group fell a total of 25%, the trazodone, fell 18% (P = NS compared to placebo), the subjects treated with Sentra PM increased 33% (P < 0.01) and the group treated with both Sentra PM and trazodone increased by 52% (P < 0.001). There was no statistical difference between placebo and trazodone. Both Sentra PM alone and combined administration showed a statistically different response compared to placebo. Both Sentra PM alone and combined administration resulted in activation of suppressed parasympathetic activity during nighttime circadian parasympathetic activity.
Table 1.
Change in parasympathetic nervous system activity shown by circadian index in each group after 14 days.
| Treatment group | Percent change from baseline to Day 14 |
|---|---|
| Placebo (S1T2) | −25.55 |
| Trazodone (S1T1) | −18.11 |
| Sentra PM (S2T1) | 33.30 |
| Sentra PM/trazodone (S2T2) | 52.65 |
Notes: The circadian index was computed at baseline and Day 14. The baseline and Day 14 circadian index was expressed as a percent change from baseline.
Figure 1.
Change in Parasympathetic Nervous System Activity in each Group after 14 Days.
Note: The circadian index is depicted for all four groups in absolute numbers from Baseline to Day 14.
Statistical Analysis
An independent biostatistician analyzed the data using the certified database. The statistical analysis was performed by methods described in Allain, et al for the LSEQ and by Jindal et al for the PSQI.48 The baseline demographics and initial measurements of the active and placebo groups were statistically compared. The HRV data was analyzed using continuous variables with the assumption of normal distributions. All randomized subjects were included, both intention to treat and completed subjects were analyzed.
Continuous variables were analyzed using t-tests and analysis of variance (ANOVA) to determine statistical differences among the four groups at entry and at the completion of treatment. Variances were tested for equivalence. When not equivalent, corrections for unequal variance were used. Contingency tables were used to analyze the dichotomous variables. A P = 0.05 was used to establish statistical significance.
Results
Of the 111 enrolled subjects, 110 completed the study. The subjects who did not complete were carried forward as an intention to treat. Uneven randomization occurred as a result of higher enrollment rates at some clinical sites however, this did not affect the statistical outcome of the study. Table 2 demonstrates the four study groups were statistically comparable on entry into the trial. Laboratory values in each of the four study groups including CBC, alkaline phosphatase (Alk Phos), Aspartate Transaminase (AST) and Alanine Transaminase (ALT) were similar at baseline.
Table 2.
Baseline measurements across all study groups.
| Treatment group | Quality | Depression | Groggy |
Mean ± SD
|
||||
|---|---|---|---|---|---|---|---|---|
| Anxiety | Awakings | Snore | Latency | Hrs slept | ||||
| Placebo | 2.15 ± 1.9 | 4.2± 3.0 | 24.9± 3.0 | 4.4 ± 3.3 | 3.4 ± 1.7 | 3.8 ± 2.9 | 83.4 ± 69.1 | 6.0 ± 7.3 |
| Trazodone | 4.39 ± 2.6 | 4.9 ± 3.7 | 27.8 ± 3.7 | 4.4 ± 3.5 | 3.8 ± 2.5 | 3.8 ± 2.2 | 62.4 ± 61.6 | 5.1 ± 2.2 |
| Sentra PM | 2.52 ± 2.7 | 3.1 ± 3.2 | 34.5 ± 3.2 | 3.0 ± 3.3 | 3.8 ± 1.6 | 4.9 ± 3.6 | 68.4 ± 46.8 | 6.5 ± 7.8 |
| Both | 2.3 ± 2.7 | 3.1 ± 2.6 | 33.6 ± 2.6 | 3.1 ± 2.9 | 3.0 ± 1.4 | 4.5 ± 2.7 | 72.3 ± 55.7 | 4.8 ± 2.1 |
Significant changes were observed between the 3 active groups and placebo after 14 days (Table 3). Sleep observations quantified by PSQI increased from 0.95 in placebo to 1.98 (NS compared to placebo) in the trazodone group, 3.86 (P < 0.01) in the Sentra PM group alone and 6.48 (P < 0.01) in the both Sentra PM and trazodone group on a 10 point PSEQ scale. The number represents the delta between the baseline and day 14 data.
Table 3.
Day 14 measurements of endpoints across each study group.
| Quality | Depression | Groggy | Anxiety | Awakings | Snore | Latency | Hrs slept | |
|---|---|---|---|---|---|---|---|---|
| Placebo | 0.95 | 1.13 | 2.8 | 1.092 | −0.76 | −0.628 | −17 | 1.46 |
| Trazodone | 1.98 NS | −0.922** | 18.9** | 0.314 NS | −1.58* | −1.45 NS | −28 NS | 1.48 NS |
| Sentra PM | 3.86** | −1.15** | −19.3** | −0.018* | −1.61* | −2.45 NS | −41* | 1.18 NS |
| Both | 6.48** | −2.3** | −28.4** | −0.155* | −0.25 | −2.77** | −56** | 2.01 NS |
| P-value | −0.001 |
Perceived morning grogginess increased 2.8 percent (LSEQ) from baseline to Day 14 in the placebo group and by 18% in the trazodone alone. By contrast, it decreased by 19.3% in the Sentra PM group and decreased by 28.4% in the group receiving both Sentra PM and trazodone. This represents a 37% difference between trazodone alone and Sentra PM alone.
Depression, as assessed by the PSEQ scale, increased by 1.13 units by the 14th day in the placebo group. In the trazodone group depression decreased by 0.93 units (P < 0.01), by 1.15 units in the Sentra PM treated group (P < 0.01) and by 2.3 units (P < 0.01) in the group treated with both trazodone and Sentra PM.
Discussion
The data from this study indicates that a medical food designed to correct nutritional deficiencies can promote more efficient and effective management of sleep disorders. Amelioration of nutritional deficiencies is an important adjunct to pharmacologic disease management especially in elderly patients who are particularly susceptible to sleep difficulties and medication side effects. Nutritional deficiencies are exacerbated in the geriatric population due to impaired protein absorption and metabolism.50 This treatment option avoids the potential for dependence on medication and reduces the side effects of sleep drugs.
Nutrient management of disease has been fundamental since the advent of therapeutic medicine. Evidence based examples of contemporary observations augmented by scientific advances is mandatory. Tepaske, et al administered an arginine based preparation to patients prior to cardiac surgery improving post-operative creatinine clearance and immune function. Fonarow, et al51,52 demonstrated improved clinical outcomes in congestive heart failure treated with amino acid neurotransmitter precursors. These two examples reinforce the opportunity that nutrient management affords clinicians. A recent study from our group demonstrated that a medical food reduced inflammation and symptoms in patients with chronic back pain.
A 50 mg dose of bedtime trazodone did not show statistically significant improvement in sleep latency, duration or quality of sleep. It did cause morning grogginess and a mild improvement in depression without impact on perceived anxiety. The medical food Sentra PM shortened time to fall asleep and improved sleep quality without morning grogginess. Sentra PM reduced depression and feelings of anxiety. Trazodone taken with the medical food produced greater improvement in all parameters compared to either trazodone or Sentra PM alone. The improvements were observed without increased morning grogginess.
Subjects in the group that received the combination of trazodone and Sentra PM responded most significantly since both interventions affect cholinergic and serotonergic pathways differently and therefore have a synergistic effect on sleep latency. The Sentra PM obviates against the trazodone side effects by providing the appropriate neurotransmitter precursors. Trazodone is then able to use more of the existing neurotransmitters rather than depleting them as it would without the medical food. This cellular technology not only improves the clinical effect but also the subject’s measured parasympathetic function. In addition, the combination of a medical food providing neurotransmitter precursors and a low dose pharmaceutical drug would decrease long term side effects and prevent attenuation of effect.
Heart rate may be altered by sympathetic activity or by parasympathetic (vagal) activity. Sympathetic and parasympathetic systems are opposite acting branches of the autonomic nervous system; this is referred to as the sympathovagal balance and is reflected in the beat-to-beat changes of the cardiac cycle.24,53,54 Beat to beat changes of the cardiac cycle can vary; this is called Heart Rate Variability (HRV). HRV can be measured by several techniques one of which is 24 hour ECG analysis. HRV measured by 24 hour ECG analysis is related to prognosis in a number of disease states such as congestive heart failure, diabetes mellitus and sleep disorders.
HRV analysis by ECG is a complex methodology. This measurement can be performed in what is called the Time Domain, measuring RR intervals in milliseconds or in the Frequency Domain using MHz and power. Time Domain HRV measurements utilize statistical methods such as mean and standard deviation of the RR-Interval. Spectral analysis of the RR tachogram measures the effect of the sympathetic and parasympathetic modulation of the RR-intervals. Spectral HRV converts the RR-Interval measurements to frequency using Fast Fourier analysis.23,24,55–58 The HRV spectrum is then divided into various spectral bands. The two main frequency bands of interest are referred to as the Low-Frequency (LF) band (0.04 to 0.15 Hz) and the High-Frequency (HF) band (0.15 to 0.4 Hz).
It is known that parasympathetic autonomic nervous system (PANS) function in normal subjects increases from midnight to 5 AM, a reflection of circadian rhythm.23,24,59,60 This increase is independent of an individual’s attempt to stay awake. The increase in PANS depends on release of acetylcholine in the brain. Patients with sleep disorders do not activate PANS during the expected circadian activation. Also, the sinus arrhythmia observed in younger people that is gradually lost with age is a well recognized manifestation of HRV. In this study, the medical food increased the subjects PANS after day 14 during the circadian activation period in both the medical food and combined with trazodone. We have previously demonstrated that another amino acid based medical food using GABA also increases circadian PANS. Thus, a medical food that provides precursors to the important parasympathetic autonomic nervous system neurotransmitter acetylcholine increases both clinical evidence of improved sleep function but also increases physiologic markers of PANS. The data provided by 24 hour HRV provides objective validation of the clinical observations.
The study of 111 subjects from 12 sites demonstrated improvement in clinical outcomes for the medical food alone or even more so in combination. It would be important to extend the study for a longer period to assess the absence of tolerance developing. Sentra PM with and without Trazodone is an alternative to conventional pharmaceutical sleep aides which have the potential for dependence and are not commonly indicated for long term use.
The ingredients in the medical food are defined by the FDA as generally recognized as safe (GRAS) and in this formulation the doses fall within the daily amount normally found in the American diet. The FDA has published extensive reviews of the safety of amino acids in both nutrient and pharmacologic doses. In addition, the FDA did not find any pharmacokinetic interaction between amino acids and pharmaceuticals. The observed superior results of the medical food taken concurrently may reflect increased availability of neurotransmitters in the relevant synapse.
Acknowledgments
During the preparation of this manuscript, Elizabeth Charuvastra passed away after a brief but brave battle with pancreatic cancer. Her contributions to this paper and to the development of safer and more effective medical treatments are incalculable. She will be sorely missed by all that have known and worked with her.
Footnotes
Author Contributions
Conceived and designed the experiments: WES, DSS, EHC, DHB. Analysed the data: WES, DSS, EHC. Wrote the first draft of the manuscript: WES, DSS. Contributed to the writing of the manuscript: WES, EHC, DHB, LAM, SLP, DSS. Agree with manuscript results and conclusions: WES, EHC, DHB, LAM, SLP, DSS. Jointly developed the structure and arguments for the paper: WES, EHC, DHB, LAM, SLP, DSS. Made critical revisions and approved final version: WES, EHC, DHB, LAM, SLP, DSS. All authors reviewed and approved of the final manuscript.
Funding
Financial support for the authors was consistent with typical consulting fees and salary for employees. None of the research on the study intervention was considered to be off-label or investigational. All study practices discussed were post-market interventional.
Competing Interests
DS and LM have received consulting fees or honoraria as a Scientific Advisory Board members of Targeted Medical Pharma. SP received payment from her employer Targeted Medical Pharma for writing or reviewing this manuscript. DB is an employee of Targeted Medical Pharma.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Reference
- 1.Foley KA, Sarsour K, Kalsekar A, Walsh JK. Subtypes of sleep disturbance: associations among symptoms, comorbidities, treatment, and medical costs. Behav Sleep Med. 2010 Apr;8(2):90–104. doi: 10.1080/15402001003622842. [DOI] [PubMed] [Google Scholar]
- 2.Parish JM. Sleep-related problems in common medical conditions. Chest. 2009 Feb;135(2):563–72. doi: 10.1378/chest.08-0934. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005 Mar;39(2):151–9. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Arnulf I, Quintin P, Alvarez JC, et al. Mid-morning tryptophan depletion delays REM sleep onset in healthy subjects. Neuropsychopharmacology. 2002 Nov;27(5):843–51. doi: 10.1016/S0893-133X(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 5.Benca RM. Consequences of insomnia and its therapies. J Clin Psychiatry. 2001;62(Suppl 10):33–8. [PubMed] [Google Scholar]
- 6.Bonnemeier H, Richardt G, Potratz J, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003 Aug;14(8):791–9. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- 7.Dworschak M, Maurer JT, Haschemian T, Rapp HJ, Waschke KF. The use of spectral measures of heart rate variability to differentiate between male snorers and patients with sleep apnoea syndrome. Anaesthesia. 2001 May;56(5):424–8. doi: 10.1046/j.1365-2044.2001.01961.x. [DOI] [PubMed] [Google Scholar]
- 8.Grazia de SM, Imeri L, De MW, Perego C, Simard S, Terrazzino S. Sleep regulation: interactions among cytokines and classical neurotransmitters. Adv Neuroimmunol. 1995;5(2):189–200. doi: 10.1016/0960-5428(95)00008-p. [DOI] [PubMed] [Google Scholar]
- 9.ncoli-Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep. 2000 Feb 1;23(Suppl 1):S23–30. [PubMed] [Google Scholar]
- 10.Saito Y. The circadian rhythm of brain acetylcholine levels and motor activity in the rat. Life Sci I. 1971 Jul 1;10(13):735–44. doi: 10.1016/0024-3205(71)90117-2. [DOI] [PubMed] [Google Scholar]
- 11.Steardo L, Bonuso S, Pisanti N, Marano E. Circadian rhythm of tryptophan in normal volunteers. Acta Neurol (Napoli) 1980 Apr;2(2):86–93. [PubMed] [Google Scholar]
- 12.Zimmermann RC, McDougle CJ, Schumacher M, et al. Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. J Clin Endocrinol Metab. 1993 May;76(5):1160–4. doi: 10.1210/jcem.76.5.8496306. [DOI] [PubMed] [Google Scholar]
- 13.Siegel JM. REM sleep: a biological and psychological paradox. Sleep Med Rev. 2011 Jun;15(3):139–42. doi: 10.1016/j.smrv.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011 Aug;15(4):269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Hauw JJ, Hausser-Hauw C, De GU, Hasboun D, Seilhean D. Neuropathology of sleep disorders: a review. J Neuropathol Exp Neurol. 2011 Apr;70(4):243–52. doi: 10.1097/NEN.0b013e318211488e. [DOI] [PubMed] [Google Scholar]
- 16.Platt B, Riedel G. The cholinergic system, EEG and sleep. Behav Brain Res. 2011 Aug 10;221(2):499–504. doi: 10.1016/j.bbr.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 17.McCarley RW. Neurobiology of REM sleep. Handb Clin Neurol. 2011;98:151–71. doi: 10.1016/B978-0-444-52006-7.00010-1. [DOI] [PubMed] [Google Scholar]
- 18.Siegel JM. The neurobiology of sleep. Semin Neurol. 2009 Sep;29(4):277–96. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008 May;31(5):673–90. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akita M, Ishii K, Kuwahara M, Tsubone H. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in guinea pigs. Exp Anim. 2002 Jan;51(1):1–7. doi: 10.1538/expanim.51.1. [DOI] [PubMed] [Google Scholar]
- 21.Barendregt PJ, Tulen JH, van den Meiracker AH, Markusse HM. Spectral analysis of heart rate and blood pressure variability in primary Sjogren’s syndrome. Ann Rheum Dis. 2002 Mar;61(3):232–6. doi: 10.1136/ard.61.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionne IJ, White MD, Tremblay A. The reproducibility of power spectrum analysis of heart rate variability before and after a standardized meal. Physiol Behav. 2002 Mar;75(3):267–70. doi: 10.1016/s0031-9384(02)00638-8. [DOI] [PubMed] [Google Scholar]
- 23.Hejjel L, Gal I. Heart rate variability analysis. Acta Physiol Hung. 2001;88(3–4):219–30. doi: 10.1556/APhysiol.88.2001.3-4.4. [DOI] [PubMed] [Google Scholar]
- 24.Malik M, Camm AJ. Heart Rate Variability. Armonk, N.Y.: Futura Publishing Company; 1995. [Google Scholar]
- 25.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther. 2002 Apr;27(2):85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 26.Sevre K, Bendz B, Hanko E, et al. Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand. 2001 Dec;173(4):409–17. doi: 10.1046/j.1365-201X.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 27.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001 Dec;10(4):253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 28.Akita M, Ishii K, Kuwahara M, Tsubone H. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in guinea pigs. Exp Anim. 2002 Jan;51(1):1–7. doi: 10.1538/expanim.51.1. [DOI] [PubMed] [Google Scholar]
- 29.Barendregt PJ, Tulen JH, van den Meiracker AH, Markusse HM. Spectral analysis of heart rate and blood pressure variability in primary Sjogren’s syndrome. Ann Rheum Dis. 2002 Mar;61(3):232–6. doi: 10.1136/ard.61.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dionne IJ, White MD, Tremblay A. The reproducibility of power spectrum analysis of heart rate variability before and after a standardized meal. Physiol Behav. 2002 Mar;75(3):267–70. doi: 10.1016/s0031-9384(02)00638-8. [DOI] [PubMed] [Google Scholar]
- 31.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther. 2002 Apr;27(2):85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 32.Sakakibara H, Luo J, Zhu SK, Hirata M, Abe M. Autonomic nervous activity during hand immersion in cold water in patients with vibration-induced white finger. Ind Health. 2002 Jul;40(3):254–9. doi: 10.2486/indhealth.40.254. [DOI] [PubMed] [Google Scholar]
- 33.Sakuragi S, Sugiyama Y, Takeuchi K. Effects of laughing and weeping on mood and heart rate variability. J Physiol Anthropol Appl Human Sci. 2002 May;21(3):159–65. doi: 10.2114/jpa.21.159. [DOI] [PubMed] [Google Scholar]
- 34.Sevre K, Bendz B, Hanko E, et al. Reduced autonomic activity during stepwise exposure to high altitude. Acta Physiol Scand. 2001 Dec;173(4):409–17. doi: 10.1046/j.1365-201X.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 35.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001 Dec;10(4):253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 36.Shell WE, Charuvastra EH, Dewood MA, May LA, Bullias DH, Silver DS. A double-blind controlled trial of a single dose naproxen and an amino acid medical food theramine for the treatment of low back pain. Am J Ther. 2010 Sep 21; doi: 10.1097/MJT.0b013e3181f4b297. [DOI] [PubMed] [Google Scholar]
- 37.Shell W, Bullias D, Charuvastra E, May LA, Silver DS. A randomized, placebo-controlled trial of an amino acid preparation on timing and quality of sleep. Am J Ther. 2010 Mar;17(2):133–9. doi: 10.1097/MJT.0b013e31819e9eab. [DOI] [PubMed] [Google Scholar]
- 38.Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966 Nov;16(11):1053–63. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- 39.Espana RA, Scammell TE. Sleep neurobiology for the clinician. Sleep. 2004 Jun 15;27(4):811–20. [PubMed] [Google Scholar]
- 40.Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101–97. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 41.Blois R, Feinberg I, Gaillard JM, Kupfer DJ, Webb WB. Sleep in normal and pathological aging. Experientia. 1983 Jun 15;39(6):551–8. doi: 10.1007/BF01971096. [DOI] [PubMed] [Google Scholar]
- 42.Inoue S. Sleep and sleep substances. Brain Dev. 1986;8(4):469–73. doi: 10.1016/s0387-7604(86)80071-7. [DOI] [PubMed] [Google Scholar]
- 43.Koella WP. The organization and regulation of sleep. A review of the experimental evidence and a novel integrated model of the organizing and regulating apparatus. Experientia. 1984 Apr 15;40(4):309–38. doi: 10.1007/BF01952538. [DOI] [PubMed] [Google Scholar]
- 44.Shell WE, Charuvastra EH, Dewood MA, May LA, Bullias DH, Silver DS. A double-blind controlled trial of a single dose naproxen and an amino acid medical food theramine for the treatment of low back pain. Am J Ther. 2010 Sep 21; doi: 10.1097/MJT.0b013e3181f4b297. [DOI] [PubMed] [Google Scholar]
- 45.Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004 Oct 1;117(7):469–78. doi: 10.1016/j.amjmed.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Shell W, Bullias D, Charuvastra E, May LA, Silver DS. A randomized, placebo-controlled trial of an amino acid preparation on timing and quality of sleep. Am J Ther. 2010 Mar;17(2):133–9. doi: 10.1097/MJT.0b013e31819e9eab. [DOI] [PubMed] [Google Scholar]
- 47.Gadde KM, Franciscy DM, Wagner HR, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003 Apr 9;289(14):1820–5. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 48.Gadde KM, Parker CB, Maner LG, et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obes Res. 2001 Sep;9(9):544–51. doi: 10.1038/oby.2001.71. [DOI] [PubMed] [Google Scholar]
- 49.Shell W, Bullias D, Charuvastra E, May LA, Silver DS. A randomized, placebo-controlled trial of an amino acid preparation on timing and quality of sleep. Am J Ther. 2010 Mar;17(2):133–9. doi: 10.1097/MJT.0b013e31819e9eab. [DOI] [PubMed] [Google Scholar]
- 50.Stover PJ. Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care. 2010 Jan;13(1):24–7. doi: 10.1097/MCO.0b013e328333d157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tepaske R, Velthuis H, Oudemans-van Straaten HM, et al. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo-controlled trial. Lancet. 2001 Sep 1;358(9283):696–701. doi: 10.1016/s0140-6736(01)05836-6. [DOI] [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. 2008 Jun 2;101(11 A):89E–103. doi: 10.1016/j.amjcard.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akita M, Ishii K, Kuwahara M, Tsubone H. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in guinea pigs. Exp Anim. 2002 Jan;51(1):1–7. doi: 10.1538/expanim.51.1. [DOI] [PubMed] [Google Scholar]
- 54.Dionne IJ, White MD, Tremblay A. The reproducibility of power spectrum analysis of heart rate variability before and after a standardized meal. Physiol Behav. 2002 Mar;75(3):267–70. doi: 10.1016/s0031-9384(02)00638-8. [DOI] [PubMed] [Google Scholar]
- 55.Akita M, Ishii K, Kuwahara M, Tsubone H. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in guinea pigs. Exp Anim. 2002 Jan;51(1):1–7. doi: 10.1538/expanim.51.1. [DOI] [PubMed] [Google Scholar]
- 56.Barendregt PJ, Tulen JH, van den Meiracker AH, Markusse HM. Spectral analysis of heart rate and blood pressure variability in primary Sjogren’s syndrome. Ann Rheum Dis. 2002 Mar;61(3):232–6. doi: 10.1136/ard.61.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dionne IJ, White MD, Tremblay A. The reproducibility of power spectrum analysis of heart rate variability before and after a standardized meal. Physiol Behav. 2002 Mar;75(3):267–70. doi: 10.1016/s0031-9384(02)00638-8. [DOI] [PubMed] [Google Scholar]
- 58.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther. 2002 Apr;27(2):85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 59.Barendregt PJ, Tulen JH, van den Meiracker AH, Markusse HM. Spectral analysis of heart rate and blood pressure variability in primary Sjogren’s syndrome. Ann Rheum Dis. 2002 Mar;61(3):232–6. doi: 10.1136/ard.61.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dionne IJ, White MD, Tremblay A. The reproducibility of power spectrum analysis of heart rate variability before and after a standardized meal. Physiol Behav. 2002 Mar;75(3):267–70. doi: 10.1016/s0031-9384(02)00638-8. [DOI] [PubMed] [Google Scholar]