Abstract
The evolution of male ornamentation often reflects compromises between sexual and natural selection, but it may also be influenced by phenotypic plasticity. We investigated the developmental plasticity of male colour ornamentation in Trinidadian guppies in response to two environmental variables that covary in nature: predation risk and food availability. We found that exposure to chemical predator cues delayed the development of pigment-based colour elements, which are conspicuous to visual-oriented predators. Predator cues also reduced the size of colour elements at the time of maturity and caused adult males to be less colourful. To the best of our knowledge, these findings provide the first example of a plastic reduction in the development of a sexually selected male ornament in response to predator cues. The influence of predator cues on ornamentation probably affects individual fitness by reducing conspicuousness to predators, but could reduce attractiveness to females. Reduced food availability during development caused males to delay the development of colour elements and mature later, probably reflecting a physiological constraint, but their coloration at maturity and later in adulthood was largely unaffected, suggesting that variation in food quantity without variation in quality does not contribute to condition dependence of the trait.
Keywords: condition dependence, male ornament, ontogeny, phenotypic plasticity, Poecilia reticulata, predator cues
1. Introduction
Developmental plasticity, or the ability of a single genotype to produce variable phenotypes depending on the developmental environment, has received attention for its adaptive value and potential role in evolutionary change [1–7]. Environmental factors have the potential to trigger alternative developmental pathways, affect the range of trait values that are expressed and alter the rate at which traits develop over time [4,8–13]. Further, developmental plasticity may either be adaptive by allowing an individual to fine tune its phenotype to environmental conditions and achieve higher fitness relative to non-plastic individuals, or non-adaptive in response to environmental perturbations or stressors that cause deviations away from the favoured phenotype [4,7–9,12,14].
Sexually selected male ornaments and displays are often particularly sensitive to the ontogenetic environment. As such, females can use male ornamentation to assess variation in male condition or quality during mate choice, given that only males in high condition can afford the cost of extreme ornamentation ([15–19], but see Cotton et al. [20]). Thus, variation in the developmental environment can not only impact the expression of sexually selected traits, with high condition males exhibiting more exaggerated ornaments [21–27], but also determine a male's probability of mating [21,28].
Sexual ornaments can also increase conspicuousness and thus, susceptibility to predation [29–35]. Displays under sexual selection have been shown to covary geographically with predation risk, suggesting that local predation pressure can interact with sexual selection to shape the evolution of these traits [36–38]. However, the degree to which sexually selected traits are plastic in response to perceived predation risk is poorly understood [28]. If plasticity is adaptive, then higher levels of perceived risk should delay the development or reduce the expression of ornaments that could attract predators [39].
Here, we examine developmental plasticity of pigment-based coloration in male Trinidadian guppies (Poecilia reticulata). Colour patterns of male guppies are considered a classic example of an ornament under opposing selection pressures; sexual selection favours elaborate colours to attract females, but natural selection favours inconspicuous colours to avoid predation [31,33,36,40–44]. High predation guppies have fewer, smaller and less elaborate colour patterns [31,36], which are produced from carotenoid and pteridine pigments (cream-white, yellow, orange and red coloration), melanin pigment (black, fuzzy black and brown coloration) and metallic, iridescent or kaleidoscopic structural patches (blues, violets, greens and silver), when compared with guppies inhabiting low predation environments [31,44–46].
Some of the variation in coloration between guppy populations, as well as a great deal of the variation within populations, has been shown to have a genetic basis [47–52], but there is also some evidence for plasticity of pigment-based coloration. Environmentally induced variation in carotenoid-based orange and yellow coloration in mature guppies has been attributed to parasitic infection and dietary content [21,45,49,53,54]. Further, melanic colour is somewhat labile, allowing black spots to dilate during courtship [40,55]. Although characteristics of predator visual systems are thought to select for different expressions of colour in male guppies [36,44], it is unknown whether predation risk also induces plastic changes in coloration. Further, the degree to which predator-induced plasticity could potentially contribute to observed variation in coloration across populations remains largely untested (see [56]).
We experimentally manipulated perceived predation risk and food quantity in the rearing environment of second-generation, laboratory-reared siblings to examine whether the pigment-based coloration of male guppies exhibits developmental plasticity. Because pigment-based colour pattern elements (hereafter, colour elements) are highly heritable and usually shared among full-sibling males [47,49], we used a split-brood design to control for genetic background and tested how the rearing environment would affect: (i) the rate at which brothers developed their shared colour elements, (ii) the number and size of colour elements at sexual maturity and (iii) overall colour expression later in adulthood. We predicted that exposure to predator cues and reduced food availability would delay development of coloration, but would not affect the number of colour elements eventually developed in adulthood, given that the basic template of colour elements is highly heritable.
2. Material and methods
(a). Field collection and laboratory methods
Trinidadian guppies were collected from a small stream, Upper La Laja (hereafter UL), in the Guanapo drainage on the southern slope of the Northern Range Mountains, Trinidad, West Indies. The UL stream lacked guppies prior to March 2008, when, as part of a separate study, guppies were translocated there from a high predation site on the Guanapo River where they coexist with a suite of predators [57], including the pike cichlid (Crenicichla frenata), which are effective visual hunters [31]. The UL site is representative of a low predation stream, because it contains only one other fish species, Rivulus hartii, a gape-limited predator on primarily juvenile guppies [57]. However, the UL site has relatively high-light conditions from experimental thinning of the forest canopy cover in July 2007 (4% canopy reduction; [58]), which potentially increased food availability [59]. In April 2009, we collected 50 juvenile guppies from the UL population and transported them to the laboratory at Colorado State University. Thus, at the time of collection, the UL population was composed of high predation individuals that had been living under low predation conditions for 1 year, or three to four generations.
To minimize maternal and other environmental effects on guppy coloration, we reared the wild-caught guppies for two generations under common garden laboratory conditions using methods modified from Reznick [60] (see the electronic supplementary material, appendix S1). Within 24 h of birth, G2 full siblings were randomly assigned to and reared in groups of two to eight siblings under one of four different rearing conditions in a 2 × 2 factorial design that varied the exposure to chemical predator cues (reared with or without predator cues) and food quantity (reared on low or high-food levels). Guppies in the predator cue treatment were reared in recirculating units that housed the common guppy predator, the pike cichlid within the sump that supplied water to the tanks [61]. Each pike cichlid was fed two guppies daily so that water flowing through each tank contained both predator kairomones and alarm pheremones, or cues that are released by guppy epidermal club cells when they are consumed [62–65]. Guppies reared without predator cues were housed in identical recirculating units without predators in the water supply. All laboratory-reared guppies were fed measured quantities of food rich in carotenoids (carotenoid pigments cannot be synthesized and must be obtained via diet [21]; electronic supplementary material, appendix S1). Guppies in the low-food treatment received roughly half of the amount of food as guppies in the high-food treatment [60].
After 29 days, when most males had not yet developed visible coloration but could be reliably sexed (see the electronic supplementary material, appendix S1), one male per rearing condition was randomly selected and reared individually thereafter, maintaining the original treatments. This design resulted in each family-line having one set of full-sibling G2 males distributed among the four different rearing environments. To quantify changes in colour during development, each male was photographed weekly beginning at day 29 and continuing until day 78 after all males had reached sexual maturity. Prior to photographing, males were anaesthetized in MS-222 (ethyl 3-aminobenzoate methane sulphonic acid salt, Sigma-Aldrich, St Louis, MO, USA) and placed laterally with their left-side facing up on a white plastic surface with their dorsal, anal and caudal fins carefully spread away from the body using a fine-tip artist paintbrush. A metric ruler was placed alongside to provide scale. A digital photograph in RAW format (unprocessed and uncompressed) was then taken using a Panasonic DMC FZ8 digital camera (Panasonic Corporation of North America, Secaucus, NJ, USA) equipped with an Opteka high definition 10× macro lens (Opteka, Inc., Drums, PA, USA) set at aperture size f11 and shutter speed 1/15 s. The illumination of males in photographs was held constant by using a single camera, no flash and lighting with two full-spectrum fluorescent lights (Philips F15T8 Natural Sunshine, 15 W, Philips Lighting Company, Andover, MA, USA), which mimic natural sunlight and were permanently fixed on either side of the camera. All images were captured at a single location in a windowless room.
Males were also anaesthetized and photographed on the day of sexual maturation, defined as the day the apical hood grew even with the tip of the gonopodium [66]. After losses from mortalities or unsuccessful crosses in the G0 and G1 generations and after excluding families with incomplete datasets, 22 G2 families (88 G2 males) were represented in the final dataset.
(b). Rate of colour development
Male guppy colour elements are typically inherited from sires in intact units or templates, and are shared among full-sibling brothers ([41,47,49], but see Gordon et al. [67]). In this study, shared colour elements were defined as distinct and contiguous areas of coloration that developed in the same basic configuration and location on each sibling, and that developed independently of adjacent colour elements (see the electronic supplementary material, figure S1). Our colour analyses were restricted to pigment-based colour elements visible to the human eye in digital photographs of anaesthetized fish. In guppies, the expression of pigment-based colour is relatively stable, except for melanic colour, which expands when fish are anaesthetized in MS-222 [40,43,55]. Although fluorescence (ultraviolet reflectance), iridescence and other structural colours are also detected by the visual systems of female guppies and predators [36,44], they were not included in our analyses because they are difficult to differentiate as discrete elements from photographs and could not be reliably scored and compared across siblings. Each family's suite of colour elements shared by all four siblings as adults at the age of 78 days was identified from day 78 photographs (see the electronic supplementary material, figure S1). Shared colour elements varied somewhat between siblings in size, intricacy, chroma and brightness, but were readily identifiable as the same colour element. Shared colour elements represented the majority (87%) of all colour elements exhibited by males by the age of 78 days and were the focus of our comparison of the rate of colour development across treatments.
To quantify the rate at which the suite of shared colour elements developed in each sibling, we then calculated the total number of shared colour elements (identified in the photos on day 78) that each sibling exhibited in each consecutive week leading up to day 78 (see the electronic supplementary material, figure S1). Weekly photos of full siblings from day 29 to 71 were analysed using a single computer and LCD monitor (Apple iMac Desktop 24-inch Mid 2007, Apple Inc., Cupertino, CA, USA) by one of us (C.L.H.) for the presence or absence of each shared colour element. We used presence/absence data to compare the rate that colour elements emerged across the different treatments. Males began to develop the first shared colour elements in the week prior to or after the first photo on day 29. Once a colour element was scored as being present, it could be reliably observed in all subsequent photographs.
We fit a nonlinear mixed model, using a logistic function in the nlme package [68] in R v. 2.15.0 [69] to statistically compare rates of development of shared colour elements across treatments (for more detail, see the electronic supplementary material, appendix S2 and [70]).
(c). Coloration and age at maturity
In addition to quantifying differences caused by treatments in the rate of development of shared colour elements, we were also interested in whether there were overall differences in the number and area of shared colour elements at the time that males reached sexual maturity. For this analysis, we used photos taken on the day that each male reached sexual maturity and we divided the shared colour elements into three readily distinguishable categories that have been used to characterize pigment-based colour: black (black and fuzzy black), orange, and yellow/cream (yellow and cream-white elements were combined into one category, because these elements occur at relatively low frequencies; [31,36,43]). We did not observe any brown or red colour elements that have been observed in other populations [36]. The areas of shared elements within each category of colour and body area were measured from each sibling's photograph at maturity by one of us (E.W.R) by outlining around colour elements and the body using the freehand tool in ImageJ v. 1.45s (http://imagej.nih.gov/ij; [43]). The sum of the areas of individual elements was used as the total area for each colour category. We did not measure colour on the dorsal fin or gonopodium, and we excluded the dorsal fin and gonopodium from measurements of body area, because they were difficult to spread consistently.
Finally, we recorded the age in days that each brother reached sexual maturity, because age at maturity could be linked to differences in the timing of colour development.
To test for differences across treatments in the age at maturity and the number and area of shared colour elements in the three colour categories, we ran Bayesian multivariate generalized linear mixed models (GLMMs) in the R package MCMCglmm [71], using R v. 2.15.1 [69] (for more detail, see the electronic supplementary material, appendix S3).
(d). Adult coloration
To assess overall adult coloration, we compared photographs of males on day 78, which was a post-maturation adult stage for all males. We counted the total number of distinct colour elements (shared and unshared) and measured body area and total area of elements assigned to the same three categories of pigment-based colour measured in the previous analysis (black, orange and yellow-white) using ImageJ.
We also had 12 naive observers, who were unfamiliar with the experimental design, compare and rank photographs of each set of four full-siblings taken on day 78 for relative overall coloration. The human eye can be used to compare more qualitative differences in overall coloration than what could be measured, using ImageJ in the analysis mentioned earlier, such as pattern sharpness and intricacy, or the enhancement of colour elements by their juxtaposition to other elements (e.g. black spots that contrast with other colour elements in guppies [41]). Observer rankings have been used to compare variation in a wide range of complex traits that are based on a combination of multiple and difficult-to-measure elements such as coloration in lizards [72] and bird plumage [13,73]. In addition, when used to compare coloration, these methods can produce results similar to those obtained by spectrophotometry within the visual range of the organism [73].
We used PowerPoint (Microsoft Corporation, Redmond, WA, USA) to arrange photographs of siblings on slides with one family represented per slide. The order of the siblings reared in each of the four conditions was randomized across families. Photographs did not contain any identifying information and were scaled so that siblings appeared to be the same length. Observers were asked to rank the four siblings on each slide from most colourful (rank = 1) to least colourful (rank = 4), based on their assessment of: (i) the number of different colours, (ii) the number of colour elements, (iii) the relative intricacy of elements, and (iv) the relative sizes and brightness of colour elements. Because fuzzy black areas can expand when guppies are exposed to MS-222 [43], we asked the observers to consider the size of black spots as the least important criterion. Each observer ranked the complete set of photographs twice with the two ranking sessions separated by 7–10 days. The intra-observer reliability was statistically significant (average Spearman's ρ = 0.48, p < 0.001 across all pairwise comparisons), and similar to the inter-observer reliability (average Spearman's ρ = 0.51, p < 0.001 across all pairwise comparisons). Thus, we calculated each fish's final observer rank as the mean of the 24 ranks by the 12 observers (the standard deviations of individuals’ mean ranks ranged from 0.00 to 1.33).
To test for differences across treatments in the total number and area of colour elements in the three colour categories and for differences in mean observer rank, we ran Bayesian multivariate GLMMs using MCMCglmm (for more detail, see the electronic supplementary material, appendix S4).
3. Results
(a). Rate of colour development
Males reared with predator cues experienced a delay in the development of shared colour elements, reaching their maximum rate of colour development (the inflection point of the logistic function) 3.9 ± 1.2 (s.e.) days later than their siblings reared without predator cues (t608 = 3.13, p = 0.002; figure 1). However, despite this delay in the age at which they reached their maximum rate of colour development, the rate itself was not affected by predator cues; in other words, the predator treatment did not affect the slope of the logistic curve at the inflection point (t608 =−0.56, p = 0.57; figure 1). Similarly, males reared on low-food levels reached their maximum rate of colour development 4.8 ± 1.2 days later than their siblings on high-food levels (t608 =−3.86, p < 0.001; figure 1) and did not differ in the rate at which colour elements were acquired at that point (t608 = 0.19, p = 0.85). The interaction between the predator cue and food quantity treatments was not significant for the inflection point (t608 =−1.26, p = 0.21) or slope of the curve at the inflection point (t608 = 0.35, p = 0.72).
Figure 1.
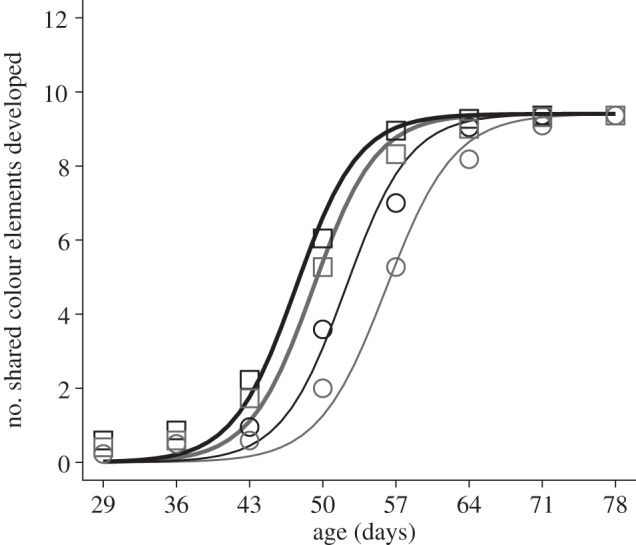
Colour development was delayed in siblings reared with predator cues and on low-food levels. The fitted functions represent the estimated fixed effects from a nonlinear mixed model including family as a random effect. These functions represent the development of colour elements that were eventually shared among all four full-siblings at day 78; the estimated asymptote was therefore constrained to be the same between treatments. Additional shared colour elements may have developed after day 78, but were not included in this analysis. Black lines represent functions for treatment combinations without predator cues and grey lines represent combinations with predator cues. Thick lines represent functions for treatment combinations with high-food levels and thin lines represent combinations with low-food levels. Squares and circles (combinations with high- and low-food levels, respectively) represent the mean number of the shared colour elements (n = 22 families, with one full-sibling reared in each of the four combinations of treatments) exhibited in weekly photos from day 29 to 78.
(b). Coloration and age at maturity
Shared black, orange, and yellow/cream colour elements were significantly smaller in males reared with predator cues at the time they reached sexual maturity (table 1); the total areas of shared black, orange, and yellow/cream colour elements were reduced by 31 per cent, 38 per cent and 31 per cent, respectively, relative to their brothers reared without predator cues. Predator cues did not significantly reduce the number of shared colour elements developed by maturity or delay the age at maturity (table 1). Low-food levels did not significantly reduce either the number or size of shared colour elements, but did delay the age at maturity by roughly 5 days (table 1).
Table 1.
Summary of the effects of the treatments on the number and area of three classes of shared colour elements (black, orange, and yellow/cream) at maturity, as well as the age at maturity (days) estimated from the posterior distributions of a Bayesian multivariate GLMM. (Family ID and body area (mm2) were included as random effects (see the electronic supplementary material, table S1 for the variance attributed to the random effects). 95% credible intervals (CIs) are included in parentheses. Estimated posterior means are shown for males reared without predator cues and on high-food levels, as well as the estimated effects of being reared with predator cues and on low-food levels. (Shared colour elements were defined as the elements that all four siblings displayed by the age of 78 days when they were all adults. The estimated level of significance (pMCMC) of each effect is indicated by asterisks: *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.)
| trait at maturity | mean without predator cues with high food (95% CI) | effect of predator cues (95% CI) | effect of low food (95% CI) | effect of predator cues × low food (95% CI) |
|---|---|---|---|---|
| number shared black elements | 1.70 (1.50, 1.88) | −0.002 (−0.29, 0.25) | 0.01 (−0.24, 0.32) | 0.00 (−0.37, 0.42) |
| area shared black elements (mm2) | 14.27 (11.06, 17.65) | −4.45 (−8.40, −0.49)* | −2.43 (−6.11, 1.87) | 2.62 (−3.22, 7.97) |
| number shared orange elements | 0.64 (0.27, 0.94) | −0.21 (−0.64, 0.29) | 0.06 (−0.42, 0.48) | −0.002 (−0.59, 0.69) |
| area shared orange elements (mm2) | 2.94 (2.48, 3.39) | −1.12 (−1.62, −0.59)*** | 0.13 (−0.40, 0.63) | −0.14 (−0.78, 0.63) |
| number shared yellow/cream elements | −0.22 (−0.71, 0.24) | −0.02 (−0.67, 0.60) | −0.07 (−0.76, 0.63) | 0.01 (−0.99, 0.92) |
| area shared yellow/cream elements (mm2) | 1.34 (1.00, 1.73) | −0.42 (−0.82, −0.04)* | −0.18 (−0.58, 0.21) | 0.13 (−0.39, 0.71) |
| age at maturity (days) | 53.08 (50.99, 55.09) | 0.97 (−1.68, 3.79) | 5.01 (2.29, 7.78)** | 2.47 (−1.71, 6.18) |
(c). Adult coloration
Quantitative assessment of coloration later in adulthood (day 78) revealed that while the total number of colour elements did not differ, males reared with predator cues had significantly smaller black and orange (but not yellow/cream) colour elements (table 2); the total areas of black and orange coloration were reduced by 21 per cent and 31 per cent, respectively, relative to their brothers reared without predator cues. Similarly, human observers ranked siblings reared with predator cues as being significantly less colourful overall (table 2). Low-food levels reduced the area of orange coloration by 13 per cent, but did not otherwise affect the number or area of colour elements. Human observers did not rank low-food males differently than their brothers reared on high food (table 2), despite the fact that they had reached sexual maturity later than their siblings.
Table 2.
Summary of the effects of the treatments on the number and area of three classes of colour elements (black, orange, and yellow/cream) and on mean observer rank when males were adults at age 78 days, estimated from the posterior distributions of a Bayesian multivariate GLMM. (Family ID and body area (mm2) were included as random effects (see the electronic supplementary material, table S2 for the variance attributed to the random effects). 95% credible intervals (CIs) are included in parentheses. Mean observer rank ranged from 1 (most colourful) to 4 (least colourful). Estimated posterior means are shown for males reared without predator cues and on high-food levels as well as the estimated effects of being reared with predator cues and on low-food levels. (The estimated level of significance (pMCMC) of each effect is indicated by asterisks: *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.)
| trait later in adulthood (78 days) | mean without predator cues with high food (95% CI) | effect of predator cues (95% CI) | effect of low food (95% CI) | effect of predator cues × low food (95% CI) |
|---|---|---|---|---|
| total number black elements | 1.89 (1.71, 2.07) | −0.06 (−0.31, 0.21) | −0.02 (−0.28, 0.25) | 0.04 (−0.30, 0.41) |
| total area black elements (mm2) | 11.43 (10.08, 12.87) | −2.39 (−3.66, −1.09)*** | −0.55 (−1.88, 0.67) | 0.71 (−0.98, 2.55) |
| total number orange elements | 1.02 (0.74, 1.29) | −0.10 (−0.48, 0.29) | −0.12 (−0.52, 0.25) | 0.05 (−0.45, 0.56) |
| total area orange elements (mm2) | 5.02 (4.42, 5.54) | −1.55 (−2.05, −1.01)*** | −0.64 (−1.15, 0.05)* | 0.55 (−0.21, 1.28) |
| total number yellow/cream elements | 0.40 (0.04, 0.77) | −0.19 (−0.74, 0.30) | 0.09 (−0.36, 0.58) | −0.16 (−0.91, 0.55) |
| total area yellow/cream elements (mm2) | 1.67 (1.24, 2.14) | −0.35 (−0.86, −0.08) | −0.02 (−0.43, 0.48) | 0.27 (−0.95, 0.38) |
| mean observer rank within family | 0.30 (−0.19, 0.84) | 1.50 (0.83, 2.15)*** | 0.27 (−0.37, 0.94) | −0.04 (−0.92, 0.98) |
4. Discussion
We found that male pigment-based coloration was developmentally plastic in response to food availability and perceived predation risk. To the best of our knowledge, this is the first evidence that the presence of predator cues in the rearing environment can delay the development and reduce the expression of a sexual ornament. This complements previous findings that predation risk can cause reductions in temporary breeding coloration in some fishes [74,75], and suggests a general role for plasticity in explaining some of the patterns of variation in sexual ornaments with predation risk observed across taxa [34,76–79] and between guppy populations. In the following, we expand on our results.
(a). Influence of predator cues
The presence of chemical cues of pike cichlid predators in the rearing environment delayed the rate of development of colour elements by almost 4 days even though maturation was not postponed, and reduced the size of those colour elements at maturity. Furthermore, perceived predation risk reduced the overall expression of coloration later in adulthood, as measured by the area of coloration and the perception of coloration by human observers, but did not affect the number of colour elements eventually developed by adult siblings. Because the males never visually observed pike cichlids, these results suggest that chemical cues alone can induce changes in coloration. The mechanisms by which colour development is delayed and coloration is reduced are not known. For example, such plastic responses could be a passive side-effect of stress induced by the presence of predator cues, however, given the central importance of colour for mate choice and predation risk it is likely that the observed changes collectively represent an adaptive strategy allowing male guppies to appear less conspicuous in an environment where predation risk is high from visual hunters. Delaying colour development and reducing the expression of coloration at maturity could increase a male's fitness by allowing him to escape predation early in life and achieve some mating success prior to becoming more conspicuous [80]. Furthermore, delayed colour development and reduced overall coloration would presumably come at the expense of attractiveness and mating success. However, this may not always be the case in high predation environments, given that the preference for colourful males can be reduced in high predation females [81,82], and female preference has been shown to switch to drabber males when females are visually exposed to predators [83,84]. By contrast, it may be beneficial to develop colour early in low predation environments in order to attract females at the onset of maturity, although colourful males may still be more susceptible to predation even in low predation environments [85]. The expression of structural colours and ultraviolet reflectance probably play a role in this balance, particularly given that pigment and structural coloration can interact under varying light conditions [44]. It is important to note that our study was limited to coloration that could be perceived by the human visual system in digital photographs from a single viewing angle, which is a subset of total coloration detected by the visual systems of the guppy and its predators [44,46].
(b). Influence of food levels
Reduced food quantity delayed the development of colour elements by almost 5 days, but this delay appeared to be more closely associated with an overall developmental delay; males reared on low-food levels matured approximately 5 days later than their brothers on high-food levels, similar to delays seen by Reznick [66]. At maturity, males reared on low-food levels showed no difference in the number or size of pigment-based colour elements. Furthermore, apart from having slightly smaller orange elements, males reared on low-food levels appeared to be as colourful as males reared on high-food levels later in adulthood. Thus, food limitation caused a delay in maturation as well as an associated delay in colour development, but eventually these males were able to achieve similar coloration as their siblings reared on higher food levels. Our results confirm those of Hughes et al. [52] that food levels alone may not affect colour patterns in adult male guppies, and that the trait is not condition-dependent with respect to food quantity (but see other studies showing effects of food quality [21,54]). Delayed development of colour patterns, in combination with delayed maturity, suggests that reduced food levels could impact male fitness. Although guppies may be able to compensate for delays caused by low-food levels early in development to achieve similar adult phenotypes as those reared on high-food levels, such compensatory growth can have negative fitness consequences later in life [86]. Furthermore, it is possible that females could reject low-food males based on other aspects of coloration not measured here (e.g. iridescence and other structural colours [44,46]) or based on other cues.
(c). Implications of plasticity of colour in the wild
Differences in the age at maturity and coloration in adult males have been shown to have a genetic basis across guppy populations. However, our results suggest that some of the variation in the size of colour elements found within and between natural populations may be due to plasticity, particularly given that predation risk probably modifies the age structure of mature males across populations [87]. High predation populations typically occur in large, low-elevation streams that are thought to have greater resource levels per guppy because their open canopy allows greater primary productivity and macro-invertebrate abundance [45,59,88], and because predation maintains guppy densities below carrying capacity [89]. By contrast, guppy populations with low predation risk typically occur in small, high elevation streams with more closed canopies and reduced food availability, where the reduced predation results in greater competition for food [89].
The UL population examined here was introduced three to four generations prior into a somewhat atypical low predation environment, given the experimentally trimmed canopy, which probably increased primary productivity overall [59]. The degree to which this population's history impacted the results observed here cannot be known until other populations are examined. However, if other populations are also plastic in response to predator cues and food availability, then plasticity probably underlies some of the differences in coloration and age at maturity observed between contrasting high and low predation environments in the wild. The laboratory environment that most closely mimicked a typical high predation environment (reared with predator cues and on high-food levels) resulted in males phenotypically similar to males found in those environments in nature (i.e. that have reduced coloration and that mature early). Similarly, the laboratory environment that mimicked a typical low predation environment (reared without predator cues and on low-food levels) resulted in males with delayed maturity and colour development, but not reduced adult colour expression. Although not investigated here, it is important to note that the gape-limited R. hartii found in low predation environments may also induce some degree of plasticity in coloration; however, its effects may be limited since R. hartii preys primarily on juvenile guppies that have not yet developed significant colour.
The plasticity documented here could play an important role when moving between environments and in adaptive evolution. Plastic genotypes should have a fitness advantage over a non-plastic genotype when encountering a range of environmental conditions [3,7]. Thus, plasticity has probably helped guppies naturally colonize a range of stream environments (e.g. predator assemblage, forest canopy cover, stream size and temperature; [59,90]). More generally, the kind of plasticity we documented here may also play an important role in the process of adaptation to novel environments [3,7,91,92]. New, changing or variable environments can alter the selective regime experienced by the genotype and reduce fitness through a mismatch between the phenotype and the environment. However, if individuals can alter the developmental rate and expression of traits in response to reliable environmental cues, then a better pairing of the phenotype and environment can be achieved.
Predator-induced plasticity in a sexual ornament has the potential to affect the evolution of female choice, particularly when females use the ornament as an indicator of male quality [93]. Differences in the developmental environment experienced by males or a genotype × environment interaction that causes males to respond differentially to environmental cues could potentially mask the honesty of the signal, and thus, interfere with the ability of females to reliably use the ornament to assess male quality [94]. This could in turn dampen the strength of sexual selection acting on the ornament itself. However, if females select among potential mates that have had a similar developmental environment and if predator cues reduce colour expression consistently across genotypes, while still allowing differences in quality to be apparent to females, then mate choice and sexual selection may be unaffected. Further study is needed to determine how plasticity in the development of sexual ornaments influences mate choice and sexual selection.
Acknowledgements
All experimental methods were approved by the Colorado State University Institutional Animal Care and Use Committee (protocols no. 03-255A-06 and 09-1348A).
We thank D. Reznick, A. López-Sepulcre, J. Feuerbacher, J. Torres-Dowdall, M. Paez, K. Langin, A. Shaw, C. Stone, K. Schnell, K. Kleinschmidt, M. Moscariello, P. Katona, R. Scudelari, S. Westrick, J. Blasius and M. Desrosiers, for their assistance with data collection. Comments from G. Grether, K. Pfennig and two anonymous reviewers improved the manuscript. This study was supported by the National Science Foundation (Frontiers in Integrative Biological Research grant no. EF-0623632 and Faculty Early Career Development grant no. DEB-0846175 to C.K.G. and Research Initiation grant no. IOS-0920622 to L. A.).
References
- 1.Robinson BW, Dukas R. 1999. The influence of phenotypic modifications on evolution: the Baldwin effect and modern perspectives. Oikos 85, 582–589 10.2307/3546709 (doi:10.2307/3546709) [DOI] [Google Scholar]
- 2.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233 10.1016/S0169-5347(03)00036-3 (doi:10.1016/S0169-5347(03)00036-3) [DOI] [Google Scholar]
- 3.Price TD, Qvarnström A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc Lond. B 270, 1433–1440 10.1098/rspb.2003.2372 (doi:10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Dewitt TJ, Scheiner SM. 2004. Phenotypic plasticity: functional and conceptual approaches. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Pigliucci M, Murren CJ, Schlichting CD. 2006. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362–2367 10.1242/jeb.02070 (doi:10.1242/jeb.02070) [DOI] [PubMed] [Google Scholar]
- 7.Ghalambor CK, Mckay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 10.1111/j.1365-2435.2007.01283.x (doi:10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 8.Smith-Gill SJ. 1983. Developmental plasticity: developmental conversion versus phenotypic modulation. Am. Zool. 23, 47–55 [Google Scholar]
- 9.Stearns SC. 1989. The evolutionary significance of phenotypic plasticity. BioScience 39, 436–445 10.2307/1311135 (doi:10.2307/1311135) [DOI] [Google Scholar]
- 10.Moran NA. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 10.1086/285369 (doi:10.1086/285369) [DOI] [Google Scholar]
- 11.Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 92–98 10.1016/0169-5347(96)81050-0 (doi:10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 12.Nijhout HF. 2003. Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18 10.1046/j.1525-142X.2003.03003.x (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- 13.Leader N, Nottebohm F. 2006. Delayed plumage maturation in socially isolated juvenile zebra finches, Taeniopygia guttata. Anim. Behav. 72, 113–121 10.1016/j.anbehav.2005.09.013 (doi:10.1016/j.anbehav.2005.09.013) [DOI] [Google Scholar]
- 14.Badyaev AV. 2005. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. B 272, 877–886 10.1098/rspb.2004.3045 (doi:10.1098/rspb.2004.3045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 16.Nur N, Hasson O. 1984. Phenotypic plasticity and the handicap principle. J. Theor. Biol. 110, 275–297 10.1016/S0022-5193(84)80059-4 (doi:10.1016/S0022-5193(84)80059-4) [DOI] [Google Scholar]
- 17.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 10.1016/S0022-5193(05)80088-8 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RA. 1995. Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. 70, 1–65 10.1111/j.1469-185X.1995.tb01439.x (doi:10.1111/j.1469-185X.1995.tb01439.x) [DOI] [PubMed] [Google Scholar]
- 19.Price TD. 2006. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 209, 2368–2376 10.1242/jeb.02183 (doi:10.1242/jeb.02183) [DOI] [PubMed] [Google Scholar]
- 20.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc Lond. B 271, 771–783 10.1098/rspb.2004.2688 (doi:10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodric-Brown A. 1989. Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav. Ecol. Sociobiol. 25, 393–401 10.1007/BF00300185 (doi:10.1007/BF00300185) [DOI] [Google Scholar]
- 22.Emlen DJ. 1997. Diet alters male horn allometry in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 264, 567–574 10.1098/rspb.1997.0081 (doi:10.1098/rspb.1997.0081) [DOI] [Google Scholar]
- 23.Cotton S, Fowler K, Pomiankowskii A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 24.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussière LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027 10.1038/nature03084 (doi:10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 25.Naguib M, Nemitz A. 2007. Living with the past: nutritional stress in juvenile males has immediate effects on their plumage ornaments and on adult attractiveness in zebra finches. PLoS ONE 2, e901. 10.1371/journal.pone.0000901 (doi:10.1371/journal.pone.0000901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp DJ. 2008. Resource-mediated condition dependence in sexually dichromatic butterfly wing coloration. Evolution 62, 2346–2358 10.1111/j.1558-5646.2008.00461.x (doi:10.1111/j.1558-5646.2008.00461.x) [DOI] [PubMed] [Google Scholar]
- 27.Gosden TP, Chenoweth SF. 2011. On the evolution of heightened condition dependence of male sexual displays. J. Evol. Biol. 24, 685–692 10.1111/j.1420-9101.2010.02205.x (doi:10.1111/j.1420-9101.2010.02205.x) [DOI] [PubMed] [Google Scholar]
- 28.Cothran RD, Stiff AR, Jeyasingh PD, Relyea RA. 2012. Eutrophication and predation risk interact to affect sexual trait expression and mating success. Evolution 66, 708–719 10.5061/dryad.r471vs43 (doi:10.5061/dryad.r471vs43) [DOI] [PubMed] [Google Scholar]
- 29.Moodie GEE. 1972. Predation, natural selection and adaptation in an unusual threespine stickleback. Heredity 28, 155–167 10.1038/hdy.1972.21 (doi:10.1038/hdy.1972.21) [DOI] [Google Scholar]
- 30.Ryan MJ, Tuttle MD, Rand AS. 1982. Bat predation and sexual advertisement in a Neotropical anuran. Am. Nat. 119, 136–139 10.1086/283899 (doi:10.1086/283899) [DOI] [Google Scholar]
- 31.Endler JA. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91 10.2307/2408316 (doi:10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 32.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438 10.1086/420412 (doi:10.1086/420412) [DOI] [Google Scholar]
- 33.Godin J-GJ, Mcdonough HE. 2003. Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav. Ecol. 14, 194–200 10.1093/beheco/14.2.194 (doi:10.1093/beheco/14.2.194) [DOI] [Google Scholar]
- 34.Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF. 2003. Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 66, 541–550 10.1006/anbe.2003.2235 (doi:10.1006/anbe.2003.2235) [DOI] [Google Scholar]
- 35.Roberts JA, Taylor PW, Uetz GW. 2007. Consequences of complex signaling: predator detection of multimodal cues. Behav. Ecol. 18, 236–240 10.1093/beheco/arl079 (doi:10.1093/beheco/arl079) [DOI] [Google Scholar]
- 36.Endler JA. 1978. A predator's view of animal color patterns. Evol. Biol. 11, 319–364 [Google Scholar]
- 37.Zuk M, Simmons LW, Cupp L. 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 33, 339–343 [Google Scholar]
- 38.Basolo AL, Wagner WE., Jr 2004. Covariation between predation risk, body size and fin elaboration in the green swordtail, Xiphophorus helleri. Biol. J. Linn. Soc. 83, 87–100 10.1111/j.1095-8312.2004.00369.x (doi:10.1111/j.1095-8312.2004.00369.x) [DOI] [Google Scholar]
- 39.Booth CL. 1990. Evolutionary significance of ontogenetic colour changes in animals. Biol. J. Linn. Soc. 40, 125–163 10.1111/j.1095-8312.1990.tb01973.x (doi:10.1111/j.1095-8312.1990.tb01973.x) [DOI] [Google Scholar]
- 40.Endler JA. 1983. Natural and sexual selection on color patterns in poeciliid fishes. Environ. Biol. Fishes 9, 173–190 10.1007/BF00690861 (doi:10.1007/BF00690861) [DOI] [Google Scholar]
- 41.Houde AE. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press [Google Scholar]
- 42.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. New York, NY: Oxford University Press [Google Scholar]
- 43.Millar NP, Reznick D, Kinnison MT, Hendry AP. 2006. Disentangling the selective factors that act on male colour in wild guppies. Oikos 113, 1–12 10.1111/j.0030-1299.2006.14038.x (doi:10.1111/j.0030-1299.2006.14038.x) [DOI] [Google Scholar]
- 44.Kemp DJ, Reznick DN, Grether GF. 2008. Ornamental evolution in Trinidadian guppies (Poecilia reticulata): insights from sensory processing-based analyses of entire colour patterns. Biol. J. Linn. Soc. 95, 734–747 10.1111/j.1095-8312.2008.01112.x (doi:10.1111/j.1095-8312.2008.01112.x) [DOI] [Google Scholar]
- 45.Grether GF, Hudon J, Endler JA. 2001. Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata). Proc. R. Soc. Lond. B 268, 1245–1253 10.1098/rspb.2001.1624 (doi:10.1098/rspb.2001.1624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemp DJ, Herberstein ME, Grether GF. 2011. Unraveling the true complexity of costly color signaling. Behav. Ecol. 23, 233–236 10.1093/beheco/arr153 (doi:10.1093/beheco/arr153) [DOI] [Google Scholar]
- 47.Winge Ø, Ditlevsen E. 1947. Colour inheritance and sex determination in Lebistes. Heredity 1, 65–83 10.1038/hdy.1947.4 (doi:10.1038/hdy.1947.4) [DOI] [Google Scholar]
- 48.Haskins CP, Haskins EF. 1951. The inheritance of certain color patterns in wild populations of Lebistes reticulatus in Trinidad. Evolution 5, 216–225 10.2307/2405461 (doi:10.2307/2405461) [DOI] [Google Scholar]
- 49.Houde AE. 1992. Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69, 229–235 10.1038/hdy.1992.120 (doi:10.1038/hdy.1992.120) [DOI] [Google Scholar]
- 50.Brooks R, Endler JA. 2001. Direct and indirect sexual selection and quantitative genetics of male traits in guppies. Evolution 55, 1002–1015 10.1554/0014-3820(2001)055[1002:DAISSA]2.0.CO;2 (doi:10.1554/0014-3820(2001)055[1002:DAISSA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 51.Karino K, Haijima Y. 2001. Heritability of male secondary sexual traits in feral guppies in Japan. J. Ethol. 19, 33–37 10.1007/s101640170015 (doi:10.1007/s101640170015) [DOI] [Google Scholar]
- 52.Hughes KA, Rodd FH, Reznick DN. 2005. Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). J. Evol. Biol. 18, 35–45 10.1111/j.1420-9101.2004.00806.x (doi:10.1111/j.1420-9101.2004.00806.x) [DOI] [PubMed] [Google Scholar]
- 53.Grether GF, Hudon J, Millie DF. 1999. Carotenoid limitation of sexual coloration along an environmental gradient in guppies. Proc. R. Soc. Lond. B 266, 1317–1322 10.1098/rspb.1999.0781 (doi:10.1098/rspb.1999.0781) [DOI] [Google Scholar]
- 54.Grether GF. 2000. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata). Evolution 54, 1712–1724 [DOI] [PubMed] [Google Scholar]
- 55.Price AC, Weadick CJ, Shim J, Rodd FH. 2008. Pigments, patterns, and fish behavior. Zebrafish 5, 297–307 10.1089/zeb.2008.0551 (doi:10.1089/zeb.2008.0551) [DOI] [PubMed] [Google Scholar]
- 56.Karim N, Gordon SP, Schwartz AK, Hendry AP. 2007. This is not déjà vu all over again: male guppy colour in a new experimental introduction. J. Evol. Biol. 20, 1339–1350 10.1111/j.1420-9101.2007.01350.x (doi:10.1111/j.1420-9101.2007.01350.x) [DOI] [PubMed] [Google Scholar]
- 57.Gilliam JF, Fraser DF, Alkins-Koo M. 1993. Structure of a tropical stream fish community: a role for biotic interactions. Ecology 74, 1856–1870 10.2307/1939943 (doi:10.2307/1939943) [DOI] [Google Scholar]
- 58.Kohler TJ, Heatherly TNI, El-Sabaawi RW, Zandonà E, Marshall MC, Flecker AS, Pringle CM, Reznick DN, Thomas SA. 2012. Flow, nutrients, and light availability influence Neotropical epilithon biomass and stoichiometry. Freshwater Sci. 31, 1019–1034 10.1899/11-141.1 (doi:10.1899/11-141.1) [DOI] [Google Scholar]
- 59.Grether GF, Millie DF, Bryant MJ, Reznick DN, Mayea W. 2001. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology 82, 1546–1559 10.1890/0012-9658(2001)082[1546:RFCCRA]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[1546:RFCCRA]2.0.CO;2) [DOI] [Google Scholar]
- 60.Reznick D. 1982. The impact of predation on life history evolution in Trinidadian guppies: genetic basis of observed life history patterns. Evolution 36, 1236–1250 10.2307/2408156 (doi:10.2307/2408156) [DOI] [PubMed] [Google Scholar]
- 61.Torres-Dowdall J, Handelsman CA, Reznick DN, Ghalambor CK. 2012. Local adaptation and the evolution of phenotypic plasticity in Trinidadian guppies (Poecilia reticulata). Evolution 66, 3432–3443 10.1111/j.1558-5646.2012.01694.x (doi:10.1111/j.1558-5646.2012.01694.x) [DOI] [PubMed] [Google Scholar]
- 62.Pfeiffer W. 1974. Pheromones in fish and amphibia. In Pheromones: frontiers of biology, vol. 32 (ed. Birch MC.), pp. 269–296 Amsterdam, The Netherlands: North Holland Publishing Company [Google Scholar]
- 63.Smith RJF. 1977. Chemical communication as adaptation: alarm substances of fish. In Chemical signals in vertebrates (eds Muller-Schwarze D, Mozell MM.), pp. 303–320 New York, NY: Plenum Press [Google Scholar]
- 64.Nordell SE. 1998. The response of female guppies, Poecilia reticulata, to chemical stimuli from injured conspecifics. Environ. Biol. Fishes 51, 331–338 10.1023/A:1007464731444 (doi:10.1023/A:1007464731444) [DOI] [Google Scholar]
- 65.Brown GE, Godin J-GJ. 1999. Chemical alarm signals in wild Trinidadian guppies (Poecilia reticulata). Can. J. Zool. 77, 562–570 10.1139/cjz-77-4-562 (doi:10.1139/cjz-77-4-562) [DOI] [Google Scholar]
- 66.Reznick DN. 1990. Plasticity in age and size at maturity in male guppies (Poecilia reticulata): an experimental evaluation of alternative models of development. J. Evol. Biol. 3, 185–203 10.1046/j.1420-9101.1990.3030185.x (doi:10.1046/j.1420-9101.1990.3030185.x) [DOI] [Google Scholar]
- 67.Gordon SP, Lopez-Sepulcre A, Reznick DN. 2012. Predation-associated differences in sex linkages of wild guppy coloration. Evolution 66, 912–918 10.5061/dryad.m4576rg5 (doi:10.5061/dryad.m4576rg5) [DOI] [PubMed] [Google Scholar]
- 68.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team 2012. nlme: linear and nonlinear mixed effects models Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 69.R Development Core Team 2012. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org/. [Google Scholar]
- 70.Sofaer HR, Chapman PL, Sillett TS, Ghalambor CK. In press Variation in nestling growth trajectories within and between populations of orange-crowned warblers: an analysis based on nonlinear mixed models. J. Avian Biol. [Google Scholar]
- 71.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–2220808728 [Google Scholar]
- 72.McCoy JK, Harmon HJ, Baird TA, Fox SF. 1997. Geographic variation in sexual dichromatism in the collared lizard, Crotaphytus collaris. Copeia 1997, 565–571 10.2307/1447560 (doi:10.2307/1447560) [DOI] [Google Scholar]
- 73.Armenta JK, Dunn PO, Whittingham LA. 2008. Quantifying avian sexual dichromatism: a comparison of methods. J. Exp Biol 211, 2423–2430 10.1242/jeb.013094 (doi:10.1242/jeb.013094) [DOI] [PubMed] [Google Scholar]
- 74.Candolin U. 1998. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc. R. Soc. Lond. B 265, 1171–1175 10.1098/rspb.1998.0415 (doi:10.1098/rspb.1998.0415) [DOI] [Google Scholar]
- 75.Kodric-Brown A. 1998. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 38, 70–81 [Google Scholar]
- 76.Slagsvold T, Dale S, Kruszewicz A. 1995. Predation favours cryptic coloration in breeding male pied flycatchers. Anim. Behav. 50, 1109–1121 (doi:10.1016/0003-3472(95)80110-3) [Google Scholar]
- 77.Huhta E, Rytkönen S, Solonen T. 2003. Plumage brightness of prey increases predation risk: an among-species comparison. Ecology 84, 1793–1799 10.1890/0012-9658(2003)084[1793:PBOPIP]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1793:PBOPIP]2.0.CO;2) [DOI] [Google Scholar]
- 78.Husak JF, Macedonia JM, Fox SF, Sauceda RC. 2006. Predation cost of conspicuous male coloration in collared lizards (Crotaphytus collaris): an experimental test using clay-covered model lizards. Ethology 112, 572–580 10.1111/j.1439-0310.2005.01189.x (doi:10.1111/j.1439-0310.2005.01189.x) [DOI] [Google Scholar]
- 79.Fowler-Finn KD, Hebets EA. 2011. The degree of response to increased predation risk corresponds to male secondary sexual traits. Behav. Ecol. 22, 268–275 10.1093/beheco/arq197 (doi:10.1093/beheco/arq197) [DOI] [Google Scholar]
- 80.Rodd HF, Sokolowski MB. 1995. Complex origins of variation in the sexual behaviour of male Trinidadian guppies, Poecilia reticulata: interactions between social environment, heredity, body size and age. Anim. Behav. 49, 1139–1159 10.1006/anbe.1995.0149 (doi:10.1006/anbe.1995.0149) [DOI] [Google Scholar]
- 81.Houde AE, Endler JA. 1990. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science 248, 1405–1408 10.1126/science.248.4961.1405 (doi:10.1126/science.248.4961.1405) [DOI] [PubMed] [Google Scholar]
- 82.Stoner G, Breden F. 1988. Phenotypic differentiation in female preference related to geographic variation in male predation risk in the Trinidad guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 22, 285–291 10.1007/BF00299844 (doi:10.1007/BF00299844) [DOI] [Google Scholar]
- 83.Godin J-GJ, Briggs SE. 1996. Female mate choice under predation risk in the guppy. Anim. Behav. 51, 117–130 10.1006/anbe.1996.0010 (doi:10.1006/anbe.1996.0010) [DOI] [Google Scholar]
- 84.Gong A, Gibson RM. 1996. Reversal of a female preference after visual exposure to a predator in the guppy, Poecilia reticulata. Anim. Behav. 52, 1007–1015 10.1006/anbe.1996.0248 (doi:10.1006/anbe.1996.0248) [DOI] [Google Scholar]
- 85.Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA. 2006. Frequency-dependent survival in natural guppy populations. Nature 441, 633–636 10.1038/nature04646 (doi:10.1038/nature04646) [DOI] [PubMed] [Google Scholar]
- 86.Auer SK, Arendt JD, Chandramouli R, Reznick DN. 2010. Juvenile compensatory growth has negative consequences for reproduction in Trinidadian guppies (Poecilia reticulata). Ecol. Lett. 13, 998–1007 [DOI] [PubMed] [Google Scholar]
- 87.Reznick DN, Butler MJ, IV, Rodd FH, Ross P. 1996. Life-history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution 50, 1651–1660 10.2307/2410901 (doi:10.2307/2410901) [DOI] [PubMed] [Google Scholar]
- 88.Reznick D, Endler JA. 1982. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36, 160–177 10.2307/2407978 (doi:10.2307/2407978) [DOI] [PubMed] [Google Scholar]
- 89.Reznick D, Butler MJ, IV, Rodd H. 2001. Life-history evolution in guppies. VII. The comparative ecology of high-and low-predation environments. Am. Nat. 157, 126–140 10.1086/318627 (doi:10.1086/318627) [DOI] [PubMed] [Google Scholar]
- 90.Torres Dowdall J, Handelsman CA, Ruell EW, Auer SK, Reznick DN, Ghalambor CK. 2012. Fine-scale local adaptation in life histories along a continuous environmental gradient in Trinidadian guppies. Funct. Ecol. 26, 616–627 10.1111/j.1365-2435.2012.01980.x (doi:10.1111/j.1365-2435.2012.01980.x) [DOI] [Google Scholar]
- 91.Schlichting C, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 92.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 10.1111/j.1420-9101.2009.01754.x (doi:10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 93.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327 10.1017/S0006323196005014 (doi:10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 94.Candolin U, Heuschele J. 2008. Is sexual selection beneficial during adaptation to environmental change? Trends Ecol. Evol. 23, 446–452 10.1016/j.tree.2008.04.008 (doi:10.1016/j.tree.2008.04.008) [DOI] [PubMed] [Google Scholar]


