Abstract
Several factors lead to expectations that the scale of larval dispersal and population connectivity of marine animals differs with latitude. We examine this expectation for demersal shorefishes, including relevant mechanisms, assumptions and evidence. We explore latitudinal differences in (i) biological (e.g. species composition, spawning mode, pelagic larval duration, PLD), (ii) physical (e.g. water movement, habitat fragmentation), and (iii) biophysical factors (primarily temperature, which could strongly affect development, swimming ability or feeding). Latitudinal differences exist in taxonomic composition, habitat fragmentation, temperature and larval swimming, and each difference could influence larval dispersal. Nevertheless, clear evidence for latitudinal differences in larval dispersal at the level of broad faunas is lacking. For example, PLD is strongly influenced by taxon, habitat and geographical region, but no independent latitudinal trend is present in published PLD values. Any trends in larval dispersal may be obscured by a lack of appropriate information, or use of ‘off the shelf’ information that is biased with regard to the species assemblages in areas of concern. Biases may also be introduced from latitudinal differences in taxa or spawning modes as well as limited latitudinal sampling. We suggest research to make progress on the question of latitudinal trends in larval dispersal.
Keywords: population connectivity, larval dispersal, pelagic larval duration, larval behaviour, genetic structure, habitat fragmentation
1. Introduction
Most bottom-associated (demersal) marine animals, including fishes, spend part of their early life as larvae in open, pelagic waters before settling into a demersal lifestyle. Pelagic larvae are subject to dispersal, and this has profound consequences for distributions, demography, genetic connectivity and management. Several factors lead to expectations that the scale and processes of larval dispersal and population connectivity1 of marine animals differ with latitude. These include contrasts in species composition and community structure, temperature influences on physiology and development, and differences in physical ocean processes. Conclusions that larval dispersal, population connectivity or a proxy thereof, differ latitudinally have been reached by influential studies, and in each case, the conclusion was that dispersal takes place over wider scales in higher latitudes. Houde [1] concluded that pelagic larval duration (PLD) is inversely associated with temperature and that fish larvae in warm seas are also more likely to starve than those in cold seas. These conclusions imply more limited larval dispersal in warm waters, because shorter PLDs are conventionally considered to lead to shorter dispersal distances (but see below), and higher mortality, owing to starvation, should reduce effective dispersal distances [2]. Based on published studies of the influence of temperature on PLD in a range of marine taxa, O'Connor et al. [3, pp. 1269–1270] concluded that ‘maximum predicted dispersal distances for larvae in colder water are much greater than those in warmer water’, and ‘population connectivity and effective population size should, in general, be inversely related to ocean temperature’. Similarly, Bradbury et al. [4], using published genetic and PLD data, concluded that dispersal distance increases with latitude: for 163 marine fish species, there were significant associations between maximum latitude, body size and genetic structure (FST). Although body size explained the most variation, this analysis revealed a weaker genetic structure at latitudes above 40°, with the largest differences at the extremes of latitude (e.g. 20° versus 60° latitude). Furthermore, research using genetic parentage and otolith microchemistry techniques in warmer waters [5] has documented dispersal in larval reef fishes over much smaller scales than have been reported from temperate waters, leading to a perception that dispersal distance is correlated with latitude. Despite these perceptions, clear examples of latitudinal differences in larval dispersal or connectivity are rare.
This review is not a meta-analysis of past work investigating temperate–tropical differences in larval dispersal: such work does not exist. Instead, the intent of this review was to examine the hypothesis of latitudinal differences in larval-fish dispersal distance, the mechanisms and assumptions underlying the hypothesis, and evidence (including commonly used proxies for larval dispersal) bearing upon it, to determine whether it is supported. We also suggest research that will be useful in testing hypotheses of latitudinal differences in larval dispersal.
Why is it important to know whether there are latitudinal differences in dispersal? Knowledge of the spatial scale of larval dispersal in marine species, a major contributor to both evolutionarily and ecologically significant population connectivity, is critical to understanding community processes ranging from biogeography to population demography, to management of fisheries and to biodiversity conservation. For example, space-based management of coastal oceans, including no-take marine reserves, is being implemented widely, and such management relies on knowing the extent and patterns of connectivity [6,7]. We know little about the fate of the increased reproduction that typically occurs inside marine reserves. This question is critical, because it addresses both the service function of reserves (e.g. export of larvae to fished areas) and the design of reserves (e.g. conservation networks connected through larval exchange [8–10]). At present, the suggestion that connectivity among marine populations might vary geographically remains untested, thus hampering the ability of managers to apply general criteria to local problems. There is often disagreement about whether evidence gathered from one geographical area (e.g. temperate coastal waters) is applicable to other geographically distinct areas (such as coral reefs).
Our focus here is on the dispersal distance of the pelagic egg and larval stage prior to settlement in demersal marine shorefishes (i.e. teleosts, the adults of which live on or near the bottom at depths less than 100 m). Because these species are relatively site-attached as adults, adult movement is unlikely to contribute greatly to either genetic or demographic connectivity. Even with this limited focus, many factors influence dispersal and connectivity, and the distance travelled is the result of biophysical processes involving hydrodynamics, as well as species-specific aspects of mortality, swimming, settlement behaviour and PLD. Although post-settlement processes modify connectivity established by movement during the pre-settlement larval phase, these are beyond the scope of this review. Note, however, that studies estimating dispersal or connectivity from settled populations (e.g. most genetic work) include influences from both larval supply and post-settlement processes, and must be interpreted with this in mind (see the electronic supplementary material). It is possible that the extent to which population connectivity is maintained by pre-settlement versus post-settlement processes varies latitudinally (T. J. Miller 2013, personal communication). Even if this is true, it is appropriate to focus on the role that larval dispersal plays, as we do here.
For the sake of clarity, we divide this review into three general classes of factors that might lead to latitudinal differences in dispersal.
(§2) Biological differences: latitudinal differences in species composition and associated characteristics (especially spawning mode and PLD) that could affect dispersal.
(§3) Physical differences: latitudinal differences in water movement and habitat fragmentation that could independently affect dispersal, regardless of the underlying species composition.
(§4) Biophysical differences: latitudinal differences in physical factors (principally temperature) that could strongly affect biological processes (such as development, swimming ability and feeding) that can, in turn, affect dispersal.
Owing to space limitations, we present details of analyses in the electronic supplementary material, and confine ourselves here to overviews of results, discussion of the implications of those results and recommendations for future research.
2. Biological differences
(a). Taxonomy and biogeography
Taxonomic composition of demersal teleost shorefishes differs with latitude at all taxonomic levels, and different orders or suborders dominate at different latitudes (for details, see the electronic supplementary material). In tropical Hawaii, eastern Pacific and Cuba, Anguilliformes, holocentroid Beryciformes, Tetraodontiformes and perciform suborders Percoidei, Blennioidei, Gobioidei, Labroidei and Acanthuroidei constitute 73–84% of the 430–700 demersal shorefish species. By contrast, in cold waters of the northwestern Atlantic, northeastern Pacific and Antarctic, Gadiformes, perciform suborders Zoarcoidei and Notothenioidei, and scorpaeniform suborders Cottoidei and Hexagrammoidei and scorpaenid genus Sebastes constitute 73 to over 90 per cent of the 55–198 species. To the extent that different taxa have different dispersal characteristics, apparent geographical differences in dispersal may simply reflect differences in faunal composition rather than differences in environments. To date, comprehensive information about taxon-specific dispersal differences is lacking, and given the non-independence of taxa and geographical distributions, it will be challenging to separate location-dependent physical and biological conditions from lineage-related factors.
(b). Taxonomy and pelagic larval duration
Longer dispersal distances are often assumed to arise from longer PLDs (e.g. [11], but see below for evaluation of this assumption). Aside from marine eels (mean PLD > 100 days), available PLD data (see the electronic supplementary material for sources and details) indicate that the orders and suborders dominating warm waters have shorter mean PLD values (23–52 days) than do taxa dominating cold waters (55–108 days: electronic supplementary material, figure S1). The generality of latitudinal trends in PLD is questionable, because these PLD values were based only on nine orders or suborders from warm waters and four from cold waters. There are also possible biases owing to habitats sampled—tropical data come mainly from shallow reefs, whereas temperate data come from a wider range of habitats (see the electronic supplementary material and §5).
(c). Spawning mode
Spawning mode (in this case, demersal eggs versus broadcast spawning with pelagic eggs) could have a strong effect on dispersal distance [4,12]. The pre-hatching period of pelagic eggs potentially increases dispersal distance, particularly in colder waters, where such periods can be weeks long [13]. This period of drift is rarely included in estimates of PLD, and it does not occur in live-bearing species or most species with demersal eggs. Further, larvae of most taxa from demersal eggs begin their pelagic larval life larger and in a more developed state than those from pelagic eggs, and the earlier acquisition of swimming ability might enable these larvae to behaviourally limit dispersal [14]. Clear latitudinal differences in spawning mode exist among taxa. In warmer locations, 60–80% of demersal shorefish species have pelagic eggs, whereas in colder locations (i.e. above 50° latitude), only 15–27% of demersal species have pelagic eggs (based on faunal lists and taxon-specific spawning modes; see the electronic supplementary material, figure S2 and table S1). Further, in most regions, larvae from demersal eggs have shorter PLDs than those from pelagic eggs (see the electronic supplementary material, and §4c, on PLD, also [4]). Spawning mode has a strong taxonomic component, with spawning modes being mostly consistent within a family. Exceptions exist, however, and in these, the trend is for taxa from higher latitudes to shift away from broadcast spawning (see the electronic supplementary material). This trend towards demersal eggs in cold waters may have implications for larval dispersal and connectivity, and highlights the need to account for spawning-mode differences in comparisons across regions.
3. Physical differences
(a). Oceanography
Latitudinal gradients in seasonality, temperature, mixed layer depth (MLD), wind and Coriolis force may potentially result in latitudinal differences in dispersal of fish larvae. The effects of physical oceanographic processes on latitudinal patterns in larval dispersal are not well discussed in the literature, and are included in few explicit, published hypotheses. Therefore, in the electronic supplementary material, we develop hypotheses about how some aspects of physical oceanography might influence latitudinal patterns of larval dispersal.
Water movement itself varies with latitude, in part, owing to changes in Coriolis force. For example, Ekman coastal upwelling should be least important at low latitudes, perhaps leading to less upwelling-cell retention in the tropics (see the electronic supplementary material). However, more energetic eddies should form at higher latitudes, and these can either advect larvae from their source, or retain them nearby, resulting in more variable larval dispersal. If the MLD is shallow, larvae may be able to vertically migrate into slower-moving water below the MLD and thereby retard dispersal. Although MLD is more stable in the tropics, it may be shallower seasonally at higher latitudes, leading to differences in larval dispersal if the MLD interacts with vertical movement of larvae as outlined.
There are clear latitudinal differences in many variables that drive coastal circulation, but, equally, there are large within-latitude regional and local differences in circulation owing to topography, coastal orientation, differences in tidal regimes, river input and a variety of other factors [15]. Although certain latitudinal trends are expected, within-latitude spatial variation may frequently override those trends, thus obscuring them (see the electronic supplementary material and §5).
(b). Habitat fragmentation
For demersal fishes with some degree of habitat association, the strength of population connections should depend not only on spatial scales of larval dispersal, but also on the scales of patchiness of benthic habitat: clearly, larvae cannot settle successfully where there is no suitable habitat, so patchiness of habitat has a direct influence on dispersal distance [16]. At coarser scales, benthic habitats for nearshore demersal species are largely determined by the spatial distribution of coastlines, found either along continental margins or around islands. Continental margins have large areas of continuous nearshore habitat, whereas islands are more isolated, with the degree of isolation depending on geographical and oceanographic distances to nearby islands or continents [17]. At finer scales, particular benthic habitats are often patchily distributed. Dispersal among patches becomes less likely as distance between suitable habitat patches increases [2].
Habitat patchiness appears to affect the scale of dispersal. A review in the recent literature, estimating demographic connectivity (see the electronic supplementary material), shows that self-recruitment (i.e. larvae settling into the same area where they were spawned) is higher along continental coastlines compared with islands (figure 1a), but this is strongly affected by the spatial scale of the study (nearly an order of magnitude larger in continuous continental coastlines compared with patchy habitats and islands; figure 1b). Controlling for spatial extent of the study, the mean scales of connectivity differ among contexts, with species in patchy habitats dispersing approximately 60–100 km, whereas species in continuous habitats dispersed approximately 900 km (figure 1c). Combined, these data suggest that larval dispersal may be more restricted in fragmented habitats.
Figure 1.
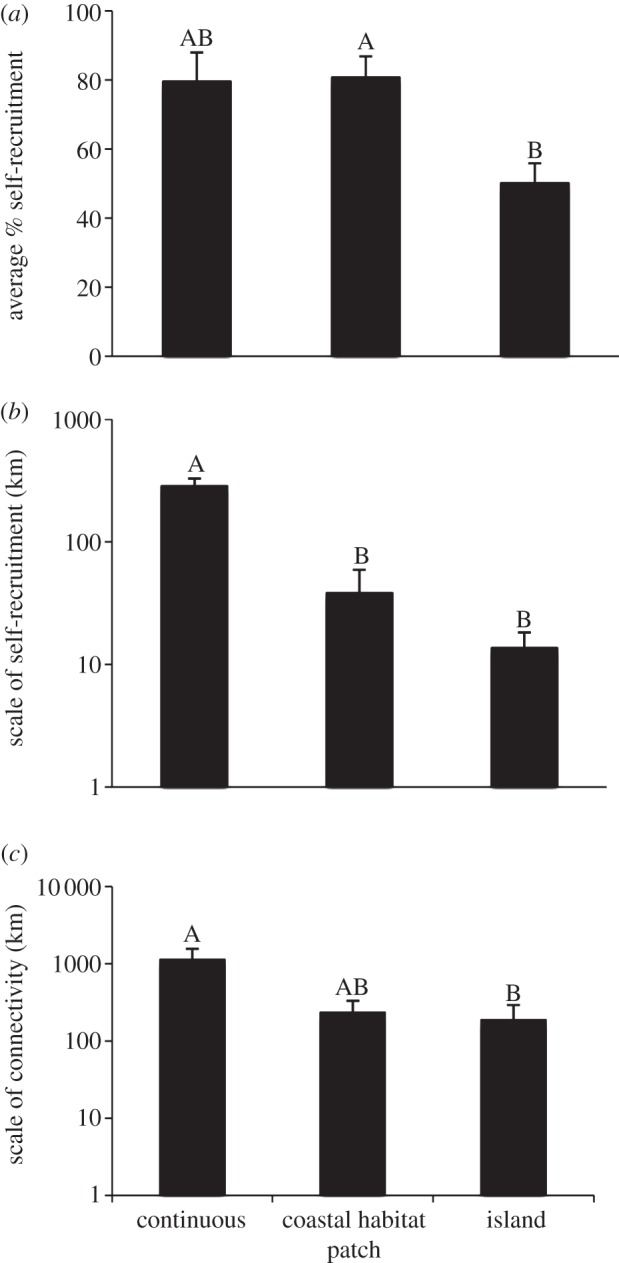
Effects of the degree of habitat patchiness based on an analysis of published otolith chemistry studies on: (a) the % of self-recruitment; (b) the scale at which self-recruitment was measured; and (c) the scale over which populations were connected. Different letters above columns indicate significant pairwise differences based on post hoc Tukey tests. Continuous refers to relatively continuous habitat on continental margins; coastal habitat patches are saltmarshes, mangroves, seagrass beds or reefs.
If habitat patchiness differs between tropical and temperate systems, then landscape context could affect dispersal. In fact, islands more than 5 km apart are two to three times more abundant in the tropics than in higher latitudes (figure 2; see electronic supplementary material), and this is expected to lead to more fragmented populations and shorter successful dispersal distances in tropical habitats. The degree of geographical isolation of habitat patches, however, may not be a consistent predictor of the likelihood of connectivity: oceanographic barriers (rather than simple distance [18–20]) or larval behaviour may modify the effect of habitat fragmentation ([21–23] and see the electronic supplementary material).
Figure 2.
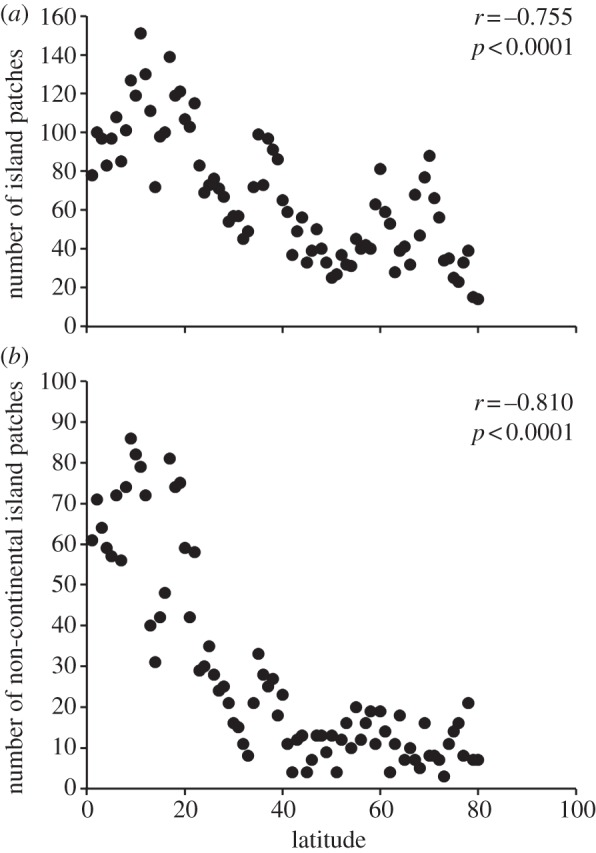
Changes in the number of: (a) all island patches and (b) non-continental island patches as a function of latitude. Each relationship was analysed by Pearson correlation. See the electronic supplementary material for details of analysis.
4. Biophysical differences
Many variables scale with latitude, including Coriolis force, seasonality and day length, but, the most obvious and important is temperature. Many of the factors considered, in this review, are temperature-related rather than latitude-related per se, but other associated factors are also important.
(a). Temperature and larval swimming
The expectation that behaviour of larvae may influence the scale of larval dispersal is based on research in three areas. First, many studies show that vertical distribution behaviour by larvae indirectly influences dispersal [14]. Second, swimming and sensory abilities of marine fish larvae are better than previously realized [14,23]: larvae of many species are able to swim directionally and at high speeds in the sea [22], which implies the ability to influence dispersal outcomes. Third, larval dispersal distances can be shorter than expected from a simple combination of advection, diffusion and PLD [5,8,24]. Combined with the growing perception that passive drift of larvae with currents could not account for this small scale [25,26], these lines of evidence have led many to presume that behaviour by larvae may restrain dispersal.
Larval swimming is expected to be constrained by temperature owing to hydrodynamic and physiological influences. For small larvae, the higher viscosity of colder water requires more swimming effort than warmer water [27], and speed is more strongly affected by viscosity than by temperature [28]. In larger larvae, effects of viscosity are reduced, but colder water should reduce metabolic rates and inhibit the motor activity required for fast swimming [27]. There is mixed support for these expectations: in the laboratory, larvae of some, but not all, species do swim faster at higher temperatures (see the electronic supplementary material).
Latitudinal comparisons of swimming performance of larvae are best made with data from laboratory studies that measure ‘critical speed’ at ambient temperatures [22,29] because more data are available for this metric. At any size, swimming speeds differed little between tropical and warm temperate species, but speeds of cold-water species were only 25–50% that of warmer water species, and their ontogenetic increase in speed was slower (details in the electronic supplementary material). Comparisons of larval-fish behaviour in situ, although hampered by the lack of data from cold temperate waters, give a somewhat different picture [22]. In situ, at any size, larvae of warm temperate species were 4–10 cm s−1 slower than tropical species, and the ratio of in situ speed to laboratory-based critical speed was larger in tropical than in warm temperate species.
The only clear latitudinal pattern in behaviour of fish larvae is that, adjusted for size, tropical and warm temperate species have similar critical speeds, and these are greater than speeds of cold temperate species. However, tropical larvae may swim faster in the sea than warm temperate species (see the electronic supplementary material). The limited evidence indicates that larvae in warm water environments swim faster and earlier in development, and this implies that larvae in lower latitudes should have more control over their dispersal. If behavioural abilities are used to restrict advection or to find settlement habitat from greater distances, then they could decrease the spatial scale of larval dispersal, a possibility supported by dispersal modelling [30], but not tested in the ocean. If so, dispersal distances in warmer waters should be smaller.
(b). Temperature, feeding and mortality
The perception exists that greater oligotrophy and higher temperatures in lower latitudes should result in more starvation of larvae [1,31], which, if true, could influence larval dispersal by slowing growth or increasing mortality (see the electronic supplementary material). Prey densities and feeding success may play a critical role in survival of pelagic larvae of marine fishes, and these factors can affect the degree to which subpopulations are connected via larval dispersal. This is because the numbers of larvae reaching any location—which affects the spatial extent of larval dispersal [15]—should be inversely related to mortality. If, however, larvae do not starve, but survive in poor condition, they may become more buoyant, and become concentrated near the surface [32]. In this case, passive larvae might be dispersed over greater distances because surface water typically moves faster than deeper water. If feeding conditions in tropical waters are indeed poorer, one might expect increased dispersal in warmer water.
Are larvae in the tropics subject to poorer feeding conditions or greater mortality from starvation? Recent literature syntheses identified latitudinal differences feeding incidence, prey types, prey selectivity and niche partitioning of fish larvae [33,34]. Feeding rates are greater in the tropics [33], and fish larvae in low and high latitudes appear similarly successful at feeding (see the electronic supplementary material), contrary to expectations. However, empirical estimates of starvation mortality are very limited [35,36], and none exist for tropical demersal species. Differences in the feeding ecologies of larval fishes between low and high latitudes are present, but little empirical evidence suggests that they result in latitudinal distinctions in dispersal or systematic geographical patterns in mortality (see the electronic supplementary material).
(c). Temperature, development and pelagic larval duration
Based solely on temperature-driven variation in physiological processes, larvae of tropical species are hypothesized to have reduced potential for dispersal owing to faster development times and shorter PLD than temperate species [3,27,37]. To test the expectation that PLD would be shorter in low latitudes, we examined PLD data for differences among latitudes (see the electronic supplementary material for details).
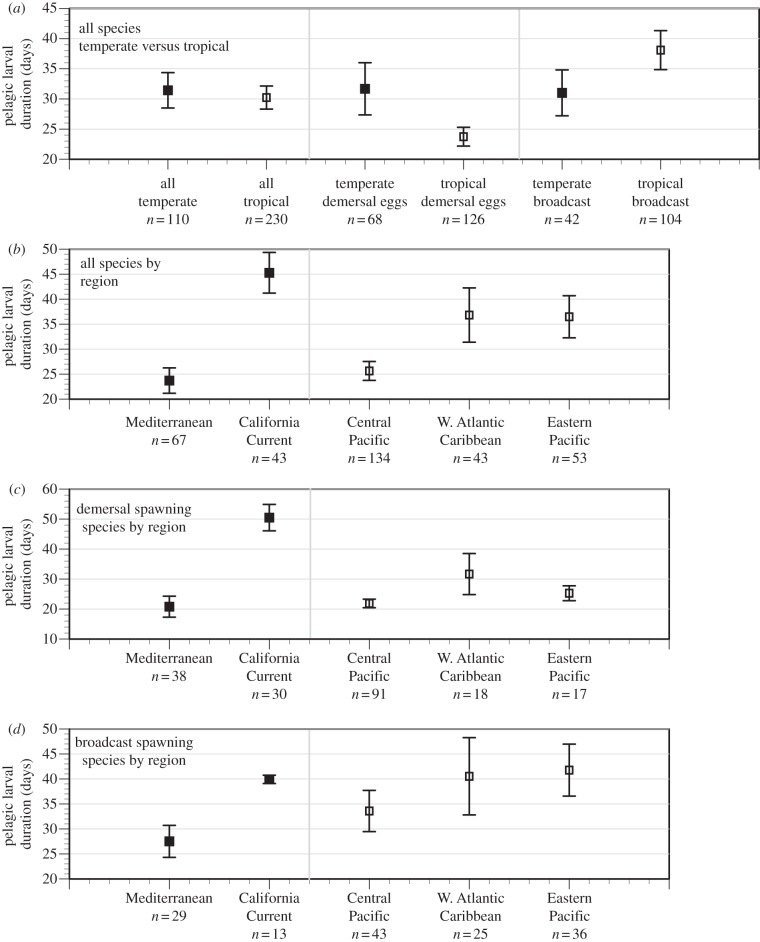
Surprisingly, regional differences in PLDs appear to be larger than differences between warm temperate and tropical sites (figure 3). These analyses, although attempting to control for habitat, reproductive mode and region, are still confounded by taxonomic influences (see the electronic supplementary material). Therefore, for the nearshore demersal species for which PLD data are available, the expectation that warm temperate PLDs were longer than tropical PLDs was not fulfilled. More comprehensive coverage of taxa and high-latitude PLD data is needed to relate PLD to latitude or temperature definitively. Finally, the relationships between PLD and other proxies for dispersal (such as genetic structure or species range) are not compelling (see the electronic supplementary material).
Figure 3.
Average (±95% CI) PLDs of temperate (solid squares) and tropical (open squares) reef fishes. (a) Data from all geographic locations and spawning modes combined and PLDs of demersal and broadcast spawning species plotted separately. (b) Data plotted by geographical region with spawning modes combined. (c) Data for demersal-spawning species plotted by geographical region. (d) Data for broadcast spawning species plotted by geographical region. If 95% CIs overlap, means are not significantly different, but if they do not overlap they are significantly different as confirmed by t-tests.
5. Discussion
The widespread view that larval dispersal and the spatial scale of population connectivity of marine fish populations differ with latitude is very plausible when theoretical considerations alone are considered. Based on either limited empirical data or these same theoretical considerations, several authors have concluded that larval dispersal probably takes place over larger scales in higher latitudes. We find only partial empirical support for this view, and the existing support is based primarily on differences in spawning mode and larval-fish behaviour between tropical and warm temperate regions versus cold temperate regions, and on habitat-fragmentation considerations.
(a). Biological differences
Existing evidence indicates that species with demersal eggs have smaller scales of genetic connectivity and generally shorter PLDs than broadcast spawners, both of which are commonly assumed to be proxies for larval dispersal distance (but see above and the electronic supplementary material for a critical evaluation of the relationship between genetics, PLD and actual dispersal distance). Most high-latitude demersal shorefish taxa are not broadcast spawners, and this should reduce the average scale of larval dispersal at high latitudes. At low-to-mid latitudes, most species are broadcast spawners, and this should increase the average scale of larval dispersal. This is contrary to the inferences drawn from habitat fragmentation data, some oceanographic variables, and the influence of temperature on physiology and behaviour. Regional differences in many factors that influence larval dispersal do exist, but the ultimate net effect of these contrasting factors on larval dispersal is far from clear: direct measures of dispersal across large geographical regions are required.
Putative latitudinal differences in spawning mode, PLD and genetic structure have been confounded by the use of data from non-representative subsets of the resident nearshore demersal fishes, biased towards pelagic spawners at high latitudes and demersal spawners at lower latitudes. However, high-latitude demersal fish assemblages are actually dominated by demersal-spawning species, whereas pelagic spawners dominate warm temperate and tropical fish assemblages. Care must be taken to ensure that questions are framed and conclusions are qualified with full regard to the mix of species for which data exist.
(b). Physical differences
In contrast to the biological and biophysical variables reviewed here, physical oceanographic variables have featured in few explicit hypotheses of latitudinal differences in larval dispersal. Although we develop several physical–oceanographic-based hypotheses in the electronic supplementary material, there is little relevant information available to test them. Water movement, the strength of upwelling and the MLD are factors that differ latitudinally, and are likely to affect the horizontal and vertical movements of larvae. Although factors affecting coastal circulation may vary over degrees of latitude, local and regional variation can also be large. Therefore, it will be difficult to determine how and to what extent physical factors may vary with latitude in their influence on larval dispersal.
Dispersal can also be affected by the frequency and spacing of suitable settlement targets, especially islands. Island habitat relative to continuous continental habitat changes along a latitudinal gradient, with more island habitat in the tropics. Thus, it is possible that tropical fishes restricted to discontinuous habitat may have shorter dispersal distances than their temperate counterparts, although empirical evidence for this is lacking.
(c). Biophysical differences
Tropical waters are warmer, and it is commonly assumed this will increase development rates: more rapid development should shorten both the pre-hatching period of pelagic eggs and PLD, and hence, it is assumed, dispersal distances. Unfortunately, the correlation between PLD and dispersal distance is weak, at best, in the species for which there are sufficient data for testing, and data suggesting shorter PLDs in the tropics are also subject to bias, because available PLD data are not a representative of the taxonomic composition or spawning modes of either tropical or temperate regions. Length of PLD is influenced not only by spawning mode, but also by adult habitat and region within the same latitudinal range, as shown here, even though our analysis is confined to nearshore demersal fishes at latitudes below 50°. Importantly, even within spawning modes, clear differences between tropical and warm temperate areas are lacking. Thus, there is no simple relationship between water temperature (or latitude) and PLD, and careful partitioning of data is required for valid latitudinal comparisons. Pelagic eggs take longer to hatch in cold water [13], and drift during this time may increase dispersal distances for broadcast spawners at high latitudes.
It is important to note that although there is ample evidence of within-species temperature-dependent responses of physiological processes related to dispersal and survival, the actual effects in nature might be minimized through adaptation of key traits. Thus, it is unclear whether well-known physiological effects of temperature actually result in geographical variation in dispersal distance or connectivity. Certainly, the strong regional and taxonomic effects on PLD (see above and the electronic supplementary material) suggest that there is wide scope for adaptation.
Larval behaviour, particularly swimming and feeding, could affect realized dispersal: both strong directed swimming and increased mortality from starvation potentially can shorten average dispersal distances. Although there is some evidence that tropical larvae swim more rapidly than temperate larvae, generalizations are difficult to make, again because of taxonomic differences and limited data from cold temperate species. Further, as with genetic and PLD data, the range of species for which larval behaviour information is available is not a representative of either the taxonomic composition or spawning modes of the assemblages from different latitudes. Equally, although there are differences in the feeding ecologies of larval fishes between low and high latitudes, there is little evidence that these differences result in latitudinal distinctions in feeding rates, starvation mortality or dispersal.
6. Future directions
There is a clear need for more studies of larval dispersal and population connectivity across latitudinal ranges. Measuring these processes empirically remains challenging. Yet, the importance of connectivity to fisheries management, conservation and predicting climate-driven changes to marine systems makes a more general understanding of latitudinal and temperature effects timely and valuable.
The various oceanographic factors considered individually here will interact in the ocean, and it is difficult to predict how they will influence dispersal when combined. Biophysical modelling that incorporates many of these oceanographic factors [38] will be helpful in understanding how latitudinal changes in physical variables influence larval dispersal.
Future latitudinal comparisons will need to take into account taxonomic composition, adult habitat and spawning mode if they are to have generality. Ideally, one would investigate a single species over large latitudinal gradients, but few species qualify. One solution is to compare species across more limited latitudinal ranges, such as sub-tropical with tropical areas. In addition, there may be cases where one could control for life history and habitat difference among higher taxonomic groupings such as the family level.
In addition, the goals for measuring connectivity must be defined clearly because these may alter the impact of any biases. For example, if the goal were fishery management or design of marine protected areas for replenishment of fished populations, a different mix of species might be appropriate to study than if the goal were biodiversity conservation or latitudinal trends in ecosystem processes. Where meta-analysis of previously published data is attempted, care must be taken to qualify interpretation and conclusions when data are biased with regard to species composition or spawning mode. Future examinations of possible latitudinal differences in larval dispersal and population connectivity will need to look beyond published data, and undertake new studies.
We must relate diet and feeding success in larvae to growth, survival and behaviour in order to understand and model how trophic-related factors ultimately affect larval dispersal and population connectivity. Linking individual-based models of larval growth and mortality to realistic circulation models could facilitate comparisons of tropical and temperate regions [39], although many of the caveats identified here will still apply, and field-testing of model predictions is required.
Currently available estimates of PLD are largely based on few individuals from very limited locations [12]. These studies have also focused on a limited range of taxonomic groups and habitats, which makes broad latitudinal comparisons problematic. It would be valuable to broaden the taxonomic base and habitats for PLD estimates, as well as to obtain better measures of within-species variation in PLD values, especially if PLD varies with location. Most PLD estimates derive from otolith counts, and because otoliths frequently do not begin to form until some time after hatching, particularly in species with pelagic eggs, many PLD values are underestimates of the true time in the water column. Better PLD estimates might reveal relationships with latitude-based factors that are not apparent with currently available estimates.
The very limited information available on larval behaviour of temperate species is another obstacle to general comparisons among areas. It would be useful to study larvae of the same species from different latitudes within its natural range when considering behaviour or effects of temperature on physiological processes to help determine the scope for adaptation. In addition, such information is needed on a broader range of species and habitats.
At present, most of the available genetic data for high latitudes are from the northern hemisphere (particularly the Atlantic), and are from larger, often pelagic, species that are of commercial interest. More single-species studies examining trends in dispersal and gene flow along latitudinal gradients are needed. Translating the observed genetic patterns into demographic trends remains challenging [40,41]. Better integration of genetic, demographic and life-history studies will be needed to further disentangle the patterns observed.
Managers are most often interested in direct measures of demographic connectivity [40,41]. Advances in otolith-based approaches and genetic-parentage approaches are being applied successfully in warmer waters, where the life histories of the fishes make these approaches particularly advantageous. They have been little applied at higher latitudes, but hold great promise.
7. Conclusions
It is important to emphasize that our conclusions apply to demersal shorefishes, and not necessarily to pelagic fishes or those from deeper waters. It is not clear that latitudinal differences in larval dispersal or associated factors exist at the level of broad faunas; certainly, they have not yet been clearly demonstrated for larvae of demersal shorefishes. This may be owing to lack of appropriate data, or the use of ‘off the shelf’ data that are biased with regard to the species assemblages in the areas of concern. Biases may be introduced from both differences in taxa or spawning modes at different latitudes as well as limited latitudinal sampling, and as we move away from ideal study types, the uncertainty increases.
Many factors lead to expectations that larval dispersal should differ latitudinally, and although most suggest broader dispersal at higher latitudes, some do the opposite. Limited evidence is available to evaluate some of these expectations, especially for higher latitudes, and for a broad array of taxa. Some hypotheses of differences are not supported by the evidence that is available on demersal shorefishes. Considerations of this issue have been dominated by untested assumptions, acceptance of logical, yet unsupported assertions and limited empirical evidence. More research on a broad array of the many factors that influence larval dispersal is required to make progress on this subject.
Acknowledgements
A.L.S. thanks Michel Kulbicki for helpful discussion and advice. Suzanne Bullock provided editorial assistance. S.D. Simpson and T.J. Miller provided helpful criticisms. T.K. was supported by the Norwegian Research Council through project MENUII no. 190286. J.M.L. was supported by ARC Discovery Grant DP110100695. J.E.C. and R.R.W. were supported by the Partnership for the Interdisciplinary Study of Coastal Oceans, funded by The David and Lucille Packard Foundation and the Gordon and Betty Moore Foundation. This is PISCO publication no. 428. J.M.L., J.E.C. and R.R.W. initiated and constructed the manuscript, provided overall editorial direction, and the introduction and conclusions. J.M.L. provided sections on taxonomy, biogeography, larval behaviour and spawning modes. I.R.B. and R.D.V. provided genetics sections. J.K.L. provided feeding sections. T.K. and C.B.P. provided physical oceanographic sections. M.J.M. provided sections on eels. M.I.O. and S.M.S. provided sections on temperature effects. A.L.S. provided PLD sections with additions from J.M.L. S.E.S. and E.A.T. provided habitat fragmentation sections. R.R.W. provided caveats sections. All contributed to the future directions section.
Endnote
For the purpose of this review, larval dispersal describes the two-dimensional distribution of larval settlement originating from a single-source population. Connectivity describes the source–destination matrix of settlers to a series of subpopulations that comprise a metapopulation connected through larval dispersal. Both terms can be spatially explicit, and are linked: short average larval dispersal distances should lead to spatially smaller metapopulations (or connectivity networks).
References
- 1.Houde ED. 1989. Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. US Fish Bull. 87, 471–495 [Google Scholar]
- 2.Cowen RK, Lwiza KMM, Sponaugle S, Paris CB, Olson DB. 2000. Connectivity of marine populations: open or closed? Science 287, 857–859 10.1126/science.287.5454.857 (doi:10.1126/science.287.5454.857) [DOI] [PubMed] [Google Scholar]
- 3.O'Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM. 2007. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl Acad. Sci. USA 104, 1266–1271 10.1073/pnas.0603422104 (doi:10.1073/pnas.0603422104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury IR, Laurel B, Snelgrove PVR, Bentzen P, Campana SE. 2008. Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B 275, 1803–1809 10.1098/rspb.2008.0216 (doi:10.1098/rspb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, van Oppen MJH, Willis BL. 2009. Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28, 307–326 10.1007/s00338-009-0469-9 (doi:10.1007/s00338-009-0469-9) [DOI] [Google Scholar]
- 6.Botsford LW, Micheli F, Hastings A. 2003. Principles for the design of marine reserves. Ecol. Appl. 13, S25–S31 10.1890/1051-0761(2003)013[0025:PFTDOM]2.0.CO;2 (doi:10.1890/1051-0761(2003)013[0025:PFTDOM]2.0.CO;2) [DOI] [Google Scholar]
- 7.Roberts CM, et al. 2003. Application of ecological criteria in selecting marine reserves and developing reserve networks. Ecol. Appl. 12(1 Suppl.), S215–S228 10.1890/1051-0761(2003)013[0215:AOECIS]2.0.CO;2 (doi:10.1890/1051-0761(2003)013[0215:AOECIS]2.0.CO;2) [DOI] [Google Scholar]
- 8.Warner RR, Cowen RK. 2002. Local retention of production in marine populations: evidence, mechanisms and consequences. Bull. Mar. Sci. 70(1 Suppl.), 245–249 [Google Scholar]
- 9.Sale PF, et al. 2005. Critical science gaps impede use of no-take fishery reserves. Trends Ecol. Evol. 20, 74–80 10.1016/j.tree.2004.11.007 (doi:10.1016/j.tree.2004.11.007) [DOI] [PubMed] [Google Scholar]
- 10.Grorud-Colvert K, et al. 2011. The assessment of marine reserve networks: guidelines for ecological evaluation. In Marine protected areas: effects, networks and monitoring—a multidisciplinary approach (ed. Claudet J.), pp. 293–321 Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.Largier JL. 2003. Considerations in estimating larval dispersal distances from oceanographic data. Ecol. Appl. 13, S71–S89 10.1890/1051-0761(2003)013[0071:CIELDD]2.0.CO;2 (doi:10.1890/1051-0761(2003)013[0071:CIELDD]2.0.CO;2) [DOI] [Google Scholar]
- 12.Riginos C, Douglas KE, Jin Y, Shanahan DF, Treml EA. 2011. Effects of geography and life history traits on genetic differentiation in benthic marine fishes. Ecography 34, 566–575 10.1111/j.1600-0587.2010.06511.x (doi:10.1111/j.1600-0587.2010.06511.x) [DOI] [Google Scholar]
- 13.Pauly D, Pullin RSV. 1988. Hatching time in spherical, pelagic marine fish eggs in response to temperature and egg size. Environ. Biol. Fishes 21, 261–271 10.1007/BF00004892 (doi:10.1007/BF00004892) [DOI] [Google Scholar]
- 14.Leis JM. 2006. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 51, 59–141 10.1016/S0065-2881(06)51002-8 (doi:10.1016/S0065-2881(06)51002-8) [DOI] [PubMed] [Google Scholar]
- 15.Cowen RK, Sponaugle S. 2009. Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 1, 443–466 10.1146/annurev.marine.010908.163757 (doi:10.1146/annurev.marine.010908.163757) [DOI] [PubMed] [Google Scholar]
- 16.Pinsky ML, Palumbi SR, Andrefouet S, Purkis SJ. 2012. Open and closed seascapes: where does habitat patchiness create populations with high fractions of self-recruitment? Ecol. Appl. 22, 1257–1267 10.1890/11-1240.1 (doi:10.1890/11-1240.1) [DOI] [PubMed] [Google Scholar]
- 17.Mora C, Treml EA, Roberts J, Crosby K, Roy D, Tittensor DP. 2012. High connectivity among habitats precludes the relationship between dispersal and range size in tropical reef fishes. Ecography 35, 89–96 10.1111/j.1600-0587.2011.06874.x (doi:10.1111/j.1600-0587.2011.06874.x) [DOI] [Google Scholar]
- 18.Barber PH, Palumbi SR, Erdmann MV, Moosa MK. 2000. Biogeography: a marine Wallace's line? Nature 406, 692–693 10.1038/35021135 (doi:10.1038/35021135) [DOI] [PubMed] [Google Scholar]
- 19.Gaylord B, Gaines SD. 2000. Temperature or transport? Range limits in marine species mediated solely by flow. Am. Nat. 155, 769–789 10.1086/303357 (doi:10.1086/303357) [DOI] [PubMed] [Google Scholar]
- 20.Taylor MS, Hellberg ME. 2003. Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science 299, 107–109 10.1126/science.1079365 (doi:10.1126/science.1079365) [DOI] [PubMed] [Google Scholar]
- 21.Kingsford MJ, Leis JM, Shanks A, Lindeman KC, Morgan SG, Pineda J. 2002. Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 70, 309–340 [Google Scholar]
- 22.Leis JM. 2010. Ontogeny of behaviour in larvae of marine demersal fishes. Ichthyol. Res. 57, 325–342 10.1007/s10228-010-0177-z (doi:10.1007/s10228-010-0177-z) [DOI] [Google Scholar]
- 23.Leis JM, Siebeck UE, Dixson DL. 2011. How Nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843 10.1093/icb/icr004 (doi:10.1093/icb/icr004) [DOI] [PubMed] [Google Scholar]
- 24.Jones GP, Srinivasan M, Almany GR. 2007. Population connectivity and conservation of marine biodiversity. Oceanography 20, 100–111 10.5670/oceanog.2007.33 (doi:10.5670/oceanog.2007.33) [DOI] [Google Scholar]
- 25.Shanks AL, Eckert G. 2005. Life-history traits and population persistence of California Current fishes and benthic crustaceans; solution of a marine drift paradox. Ecol. Monogr. 75, 505–524 10.1890/05-0309 (doi:10.1890/05-0309) [DOI] [Google Scholar]
- 26.Shanks AL. 2009. Pelagic larval duration and dispersal distance revisited. Biol. Bull. Mar. Biol. Lab. Woods Hole 216, 373–385 [DOI] [PubMed] [Google Scholar]
- 27.Hunt von Herbing I. 2002. Effects of temperature on larval fish swimming performance: the importance of physics to physiology. J. Fish Biol. 61, 865–876 10.1111/j.1095-8649.2002.tb01848.x (doi:10.1111/j.1095-8649.2002.tb01848.x) [DOI] [Google Scholar]
- 28.Fuiman LA, Batty RS. 1997. What a drag it is getting cold: partitioning the physical and physiological effects of temperature on fish swimming. J. Exp. Biol. 200, 1745–1755 [DOI] [PubMed] [Google Scholar]
- 29.Leis JM, Balma P, Ricoux R, Galzin R. 2012. Ontogeny of swimming ability in the European Sea Bass, Dicentrarchus labrax (L.) (Teleostei: Moronidae). Mar. Biol. Res. 8, 265–272 10.1080/17451000.2011.616898 (doi:10.1080/17451000.2011.616898) [DOI] [Google Scholar]
- 30.Cowen RK, Paris CB, Sirnivasan A. 2006. Scaling of connectivity in marine populations. Science 311, 522–527 10.1126/science.1122039 (doi:10.1126/science.1122039) [DOI] [PubMed] [Google Scholar]
- 31.Doherty PJ, Williams DM, Sale PF. 1985. The adaptive significance of larval dispersal in coral reef fishes. Environ. Biol. Fishes 12, 81–90 10.1007/BF00002761 (doi:10.1007/BF00002761) [DOI] [Google Scholar]
- 32.Sclafani M, Taggart CT, Thompson KR. 1993. Condition, buoyancy and the distribution of larval fish: implications for vertical migration and retention. J. Plankton Res. 15, 413–435 10.1093/plankt/15.4.413 (doi:10.1093/plankt/15.4.413) [DOI] [Google Scholar]
- 33.Llopiz JK. 2013. Latitudinal and taxonomic patterns in the feeding dynamics of fish larvae: a literature synthesis. J. Mar. Syst. 109–110, 69–77 10.1016/j.jmarsys.2012.05.002 (doi:10.1016/j.jmarsys.2012.05.002) [DOI] [Google Scholar]
- 34.Peck MA, Huebert KB, Llopiz JK. 2012. Intrinsic and extrinsic factors driving match-mismatch dynamics during the early life history of marine fishes. Adv. Ecol. Res. 47, 177–302 10.1016/B978-0-12-398315-2.00003-X (doi:10.1016/B978-0-12-398315-2.00003-X) [DOI] [Google Scholar]
- 35.Gronkjaer P, Clemmesen C, St John M. 1997. Nutritional condition and vertical distribution of Baltic cod larvae. J. Fish. Biol. 51, 352–369 10.1111/j.1095-8649.1997.tb06108.x (doi:10.1111/j.1095-8649.1997.tb06108.x) [DOI] [Google Scholar]
- 36.Tanaka Y, Satoh K, Yamada H, Takebe T, Nikaido H, Shiozawa S. 2008. Assessment of the nutritional status of field-caught larval Pacific bluefin tuna by RNA/DNA ratio based on a starvation experiment of hatchery-reared fish. J. Exp. Mar. Biol. Ecol. 354, 56–64 10.1016/j.jembe.2007.10.007 (doi:10.1016/j.jembe.2007.10.007) [DOI] [Google Scholar]
- 37.Munday PL, Leis JM, Lough JM, Paris CB, Kingsford MJ, Berumen ML, Lambrechts J. 2009. Climate change and coral reef connectivity. Coral Reefs 28, 379–395 10.1007/s00338-008-0461-9 (doi:10.1007/s00338-008-0461-9) [DOI] [Google Scholar]
- 38.Gallego A, North EW, Petitgas P, Browman HI. 2007. Advances in modelling physical-biological interactions in fish early life history. Mar. Ecol. Prog. Ser. 347, 121–306 10.3354/meps06972 (doi:10.3354/meps06972) [DOI] [Google Scholar]
- 39.Paris CB, Cherubin LM, Cowen RK. 2007. Surfing, spinning, or diving from reef to reef: effects on population connectivity. Mar. Ecol. Prog. Ser. 347, 285–300 10.3354/meps06985 (doi:10.3354/meps06985) [DOI] [Google Scholar]
- 40.Lowe WH, Allendorf FW. 2010. What can genetics tell us about population connectivity? Mol. Ecol. 19, 3038–3051 10.1111/j.1365-294X.2010.04688.x (doi:10.1111/j.1365-294X.2010.04688.x) [DOI] [PubMed] [Google Scholar]
- 41.Leis JM, van Herwerden L, Patterson HM. 2011. Estimating connectivity in marine fish populations: what works best? Oceanogr. Mar. Biol. Annu. Rev. 49, 193–234 [Google Scholar]