Fig. 3.
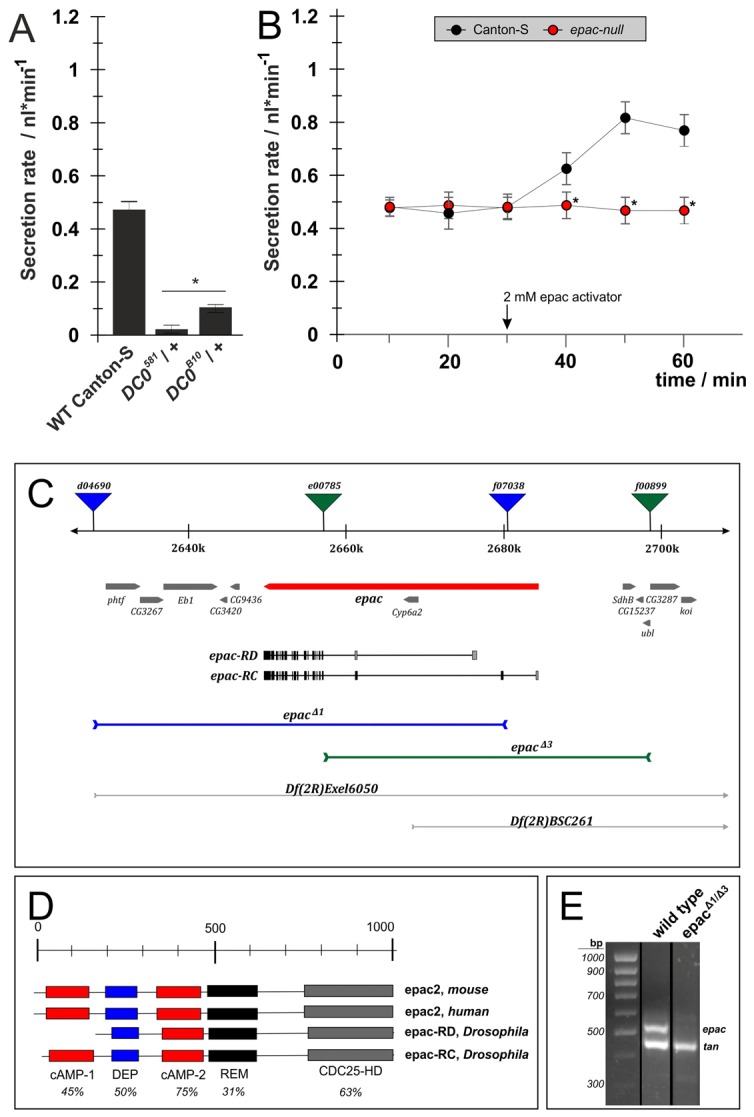
The cAMP effectors PKA and EPAC have separable effects on fluid secretion. For manipulation of PKA we used different alleles of the PKA catalytic subunit DC0 isolated by Kalderon and Rubin (Kalderon and Rubin, 1988). For manipulation of the non-canonical cAMP target EPAC we generated a null EPAC mutant by targeted deletion. (A) Basal fluid secretion rates in DC0581 or DC0B10 heterozygotes were strongly reduced compared with wild-type Canton-S controls. (B) In epac null tubules, basal secretion was indistinguishable from wild-type Canton-S controls. However, they failed to respond to application of 2 mM 8-pCPT-2′-O-Me-cAMP, a cell-permeable, EPAC-specific agonist. All data are means ± s.e.m. (n>20) from two independent experiments. Significant (P<0.05) differences are denoted by asterisks (Student's t-test, two-tailed). (C) Deletions at the unique Drosophila epac locus were generated by remobilization of FRT-containing P-elements: epacΔ1 was generated by combining d04690 and f07038; epacΔ3 by combining e00785 and f00899. (D) Homology comparison of epac proteins from human, mouse and Drosophila indicates high homology. (E) QRT-PCR on cDNA generated from either epacΔ1/epacΔ3 or wild-type Canton-S flies. Specific primers were used to amplify products from either Drosophila epac or tan, which served as an internal control.
