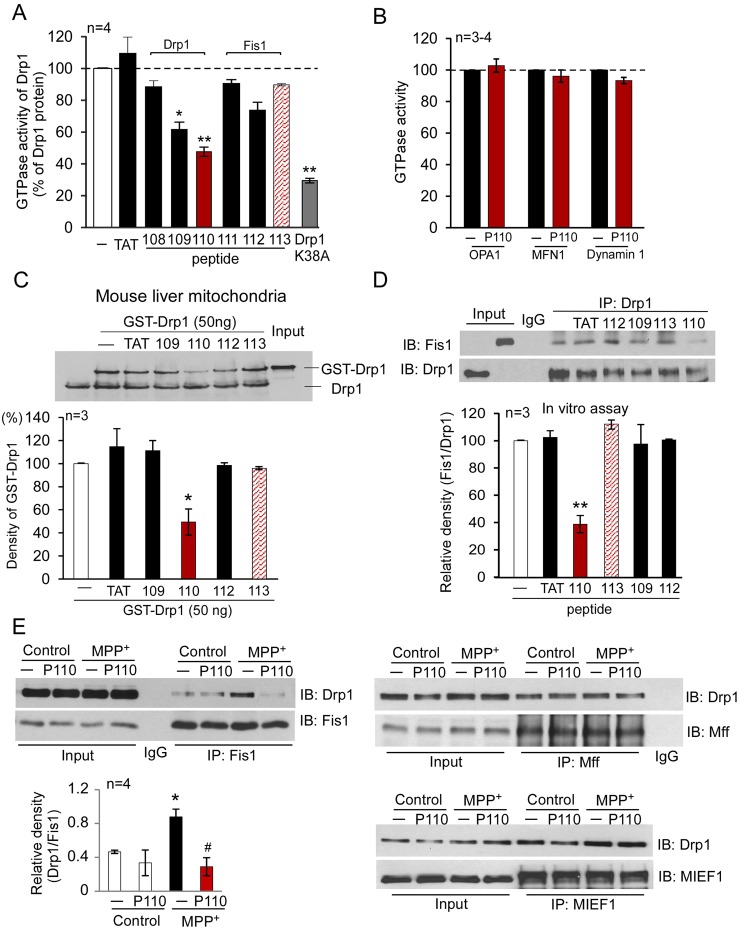
Fig. 2.
Characterization of the Fis1- and Drp1-derived peptides in vitro. (A) GTPase activity of Drp1 was determined using Drp1 recombinant protein (GST-Drp1, 25 ng) in the presence or absence of peptides P108, P109, P110, P111, P112 and P113 (1 µM each). Peptides P108–P110 were derived from human Drp1 and peptides 111–113 were from Fis1, as indicated in Fig. 1, and conjugated to the cell-permeable peptide TAT47–57. TAT47–57 was used as a control peptide carrier, and Drp1K38A (25 ng) dominant negative was used as a positive control. The data are expressed as means ± s.e. of four independent experiments (*P<0.05; **P<0.01 versus Drp1 recombinant protein alone). (B) GTPase activities of human MFN1, OPA1 and dynamin-1 (25 ng each) were determined in the presence or absence of peptide P110 (1 µM). (C) Mitochondria-enriched fraction (100 µg mitochondrial protein) was isolated from mouse liver and incubated with GST-Drp1 (50 ng) in the presence of the indicated peptides. Upper panel: western blot analysis of GST-Drp1 association with the mitochondrial fraction using anti-Drp1 antibodies. Lower panel: histograms depicting the amounts of GST-Drp1 associated with the mitochondria of mouse liver. The data are expressed as means ± s.e. of three independent experiments; *P<0.05 versus control group. (D) P110 blocked the interaction between Drp1 and Fis1. Peptides P109, P112, P110 and P113 (1 µM, each) or peptide carrier, TAT47–57 (1 µM), were incubated with Drp1 recombinant proteins (100 ng) for 30 minutes prior to the addition of Fis1 recombinant protein (100 ng). The mixed proteins were treated with a chemical cross-linker DSP (1 mM for 30 min). Immunoprecipitates (IP) with anti-Drp1 antibodies were analyzed by immunoblotting (IB) with anti-Fis1. Quantitative data are provided in the histogram. The data are expressed as means ± s.e. of three independent experiments; **P<0.01 versus control group. (E) Human SH-SY5Y neuronal cells were treated with the indicated peptides (1 µM) for 1 hour followed by treatment with MPP+ (2 mM for 1 hour). Following a brief in vivo cross-linking, cells were homogenized. Total cell lysates were then subjected to immunoprecipitation (IP) with anti-Fis1, anti-Mff or anti-MIEF1 antibodies, respectively, and the immunoprecipitates were analyzed by immunoblotting (IB) with anti-Drp1 antibodies. Quantitative data are provided in the histogram. The data are expressed as means ± s.e. of four independent experiments; *P<0.05 versus control group; #P<0.05 versus MPP+-treated group. Input lanes in D and E represent 10% of the sample used for immunoprecipitation.