Fig. 8.
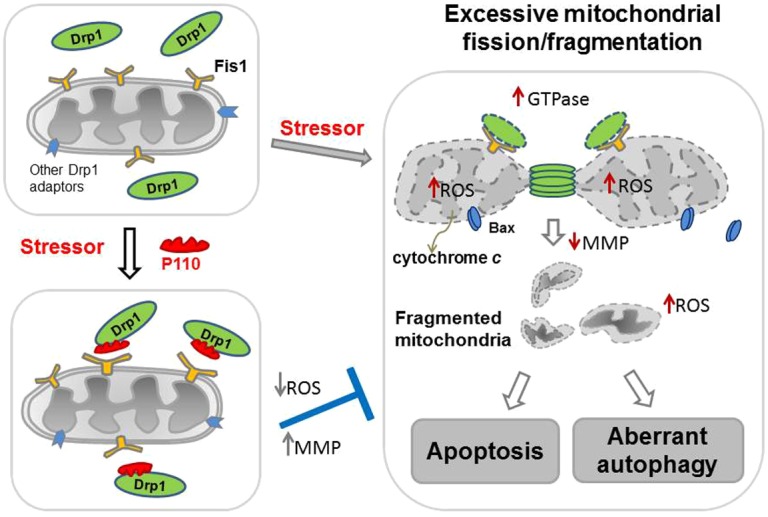
Summary scheme. Under normal conditions (upper left-hand panel), Drp1 (green) is located mostly in the cytosol. Following application of a variety of cell stressors (right-hand panel), Drp1 translocates to the mitochondria. The mitochondria-associated Drp1 has increased GTPase activity. On the mitochondrial surface, Drp1 binds to its mitochondrial adaptors, such as Fis1, a second component of the mitochondrial fission machinery, under stress conditions, and wraps around the mitochondrial tubules to drive mitochondrial division (fission). Under stress conditions, association of Drp1 with the mitochondria causes an increase in mitochondrial ROS production, which subsequently leads to dissipation of mitochondrial membrane potential (MMP). As a consequence, Bax, a pro-apoptotic factor, translocates from the cytosol to the mitochondria and the mitochondrial inter-membrane space protein, cytochrome c, is released to the cytosol, leading to increased apoptosis. Increased mitochondrial ROS production and decreased MMP also lead to excessive mitochondria-related autophagy, another type of cell death. Drp1-derived peptide inhibitor, P110 (red), binds to Drp1 and inhibits Drp1's GTPase activity and association with the mitochondria (lower left-hand panel), probably by blocking the interaction between Drp1 and Fis1 on the mitochondria. P110 treatment thus prevents the Drp1-induced cascade of mitochondria-induced excessive fission and cell death by inhibiting the translocation of Drp1 to the mitochondria.
