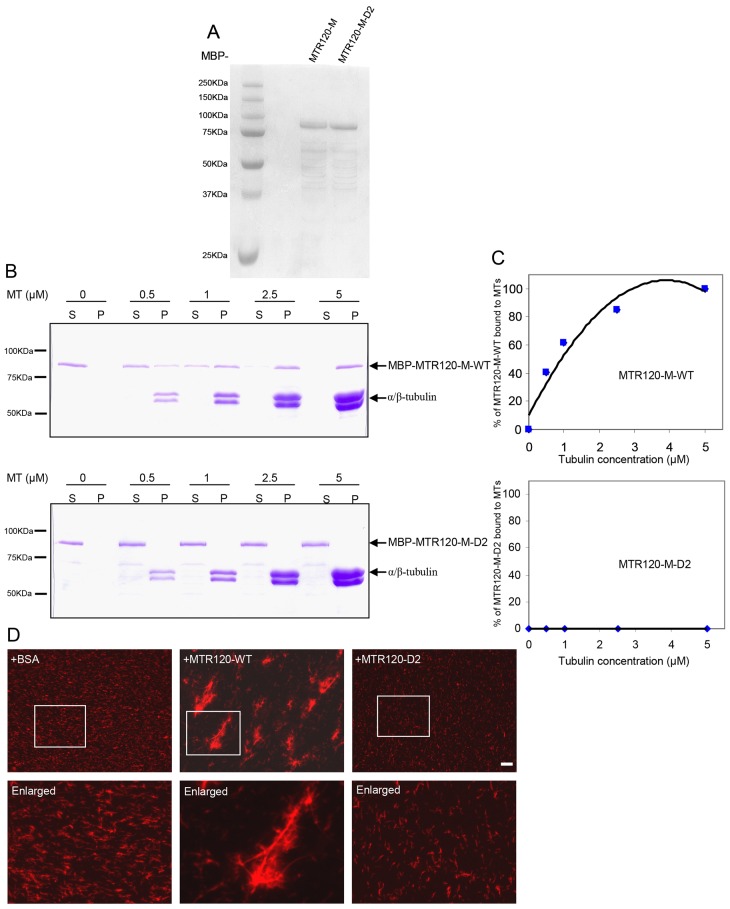
Fig. 5.
MTR120 binds to and stabilizes MTs in vitro. (A) MBP-tagged fusion proteins were purified from E. coli, separated by SDS-PAGE and stained with Coomassie Blue. (B) We incubated 200 nM of the indicated proteins with 0, 0.5, 1, 2.5 or 5 µM paclitaxel-stabilized MTs and subjected them to ultracentrifugation to pellet the polymerized MT. The supernatant and pellet fractions were run on 10% polyacrylamide gel and stained using Coomassie Blue. (C) Quantitative analysis was performed of the binding properties between MTs and MTR120-M-WT or MTR120-M-D2. The percentages of bound protein were plotted against tubulin concentrations. The disassociation constant (Kd) was determined from the best-fit curve. The data were collected from three independent experiments. (D) An MT bundling assay was performed using 4 µM rhodamine-labeled MTs. We used 0.1 µM BSA (as the negative control), wild-type (WT) or the D2 mutant MTR120-SFB. Boxed areas are enlarged. Scale bars: 10 µm.