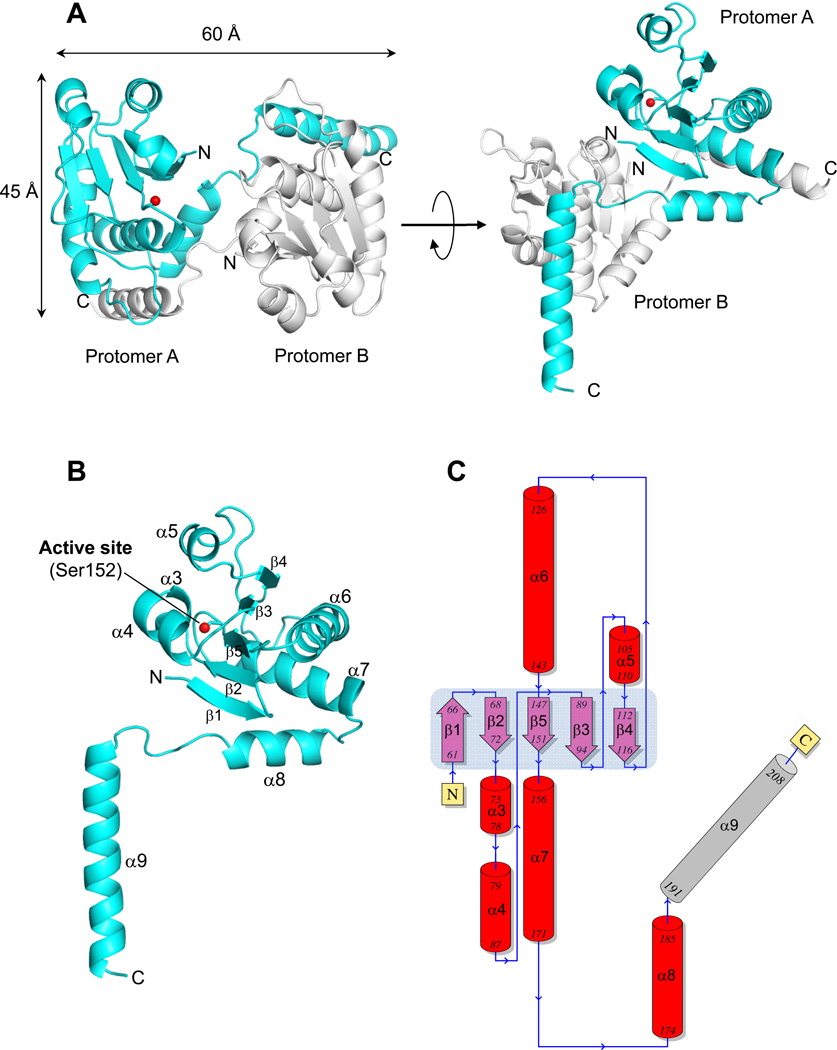
Figure 3. Atomic structure of ΔN60-C/S-DUSP26 at 1.68 Å resolution.
(A) Ribbon diagram of ΔN60-C/S-DUSP26 crystallographic dimer (in side and top view) that is present in two copies in the asymmetric unit. Two protomers of a dimer (referred to as A and B) are colored in cyan and gray, respectively. The position of protomer A catalytic residue (Ser152) is shown as a red ball. (B) Ribbon diagram of protomer A showing all secondary structure element. (C) Topological diagram of ΔN60-C/S-DUSP26 protomer A with α-helices and β-strands forming the DSP-core colored in red and purple, respectively and the domain swapped helix α9 in gray. The central β-sheet formed by strands β1–β5 is highlighted in light blue. A complete list of crystallographic parameters is in Table 2.