Abstract
OBJECTIVE
Previous experiments in Yorkshire swine demonstrated significantly fewer pericardial adhesions and intramyocardial collagen deposition at reoperative sternotomy in animals supplemented with vodka but not with red wine. The purpose of this experiment was to determine a mechanism for adhesion reduction.
METHODS
Twenty-seven male Yorkshire swine were fed a high-cholesterol diet to simulate conditions of coronary artery disease followed by the surgical placement of an ameroid constrictor to the left circumflex coronary artery to induce chronic ischaemia. Postoperatively, control pigs continued their high-fat/cholesterol diet alone, whereas the two experimental groups had diets supplemented with either red wine or vodka for 7 weeks followed by reoperative sternotomy and cardiac harvest.
RESULTS
The expression of related adhesion focal tyrosine kinase (RAFTK) and caspase 3 in the sodium dodecyl sulphate (SDS)-soluble myocardial fraction was significantly higher only in the vodka-supplemented group. In the more soluble fraction, the expression of caspase 3, cleaved caspase 3 and caspase 9 was lower in both the vodka and red wine treatment groups.
CONCLUSIONS
In the SDS-soluble lysate fraction, likely representing the transmembrane/cell–extracellular matrix (ECM), a significant increase in RAFTK and caspase 3 expression was seen only in the vodka-treated animals, which may explain why this group demonstrated significantly fewer pericardial adhesions. Caspase expression/signalling was not increased in the more soluble myocardial lysate, suggesting that the increased apoptotic signalling was specific to the epicardial–ECM.
Keywords: Pericardial adhesions, Alcohol, Ischaemia, Caspase, Apoptosis
INTRODUCTION
Adhesions encountered during reoperative cardiac surgery can greatly increase the difficulty of the second operation. These fibrinous attachments of the epicardium and pericardium distort anatomy and tissue planes which can lead to longer operative times, increased bleeding and potentially worse clinical outcomes as well as increased costs. Reoperative cardiac surgery can be particularly challenging if the second procedure occurs within a few weeks of the initial operation, when adhesions are most dense and surrounded by thickened, inflamed pericardial tissues. Thus, recent animal studies in both the academic and biomedical industry sectors have attempted to develop methods to reduce the formation of adhesions after cardiac surgery. These studies have focused largely on the local application of products and substrates such as bioabsorbable polymer film barriers, and the application of growth factors and collagen membranes directly into the surgical field at the time of initial operation [1–3]. Though the local application of products and substrates does reduce the risk of unwanted systemic side effects, most surgeons would prefer not to place foreign materials into a surgical field before closing, in part due to the increased risk of infection and potential immune reaction to foreign material. On the other hand, systemic anti-inflammatory treatments such as steroids or other immune modulators carry non-site-specific and other unwanted side effects, limiting their use in the prevention of adhesion formation. Thus, the systemic administration of a substance with desired, or at least no undesirable, non-site-specific effects as well as local adhesiolytic properties would be most appealing for use in the prevention of postoperative pericardial adhesions. Research in systemic anti-adhesion treatments is emerging, and recent studies in a rabbit model have demonstrated the systemic administration of sunitinib, a monoclonal antibody used in the treatment of various solid tumours, to significantly reduce postoperative adhesions without any immediate, undesired systemic effects [4]. Alcohol consumption in humans spans every socioeconomic class and has numerous known health benefits as well as known roles in several disease processes. In regard to cardiac and cardiovascular heath, moderate alcohol consumption is widely regarded to be beneficial, and in fact, cardioprotective in individuals when compared with non-drinkers or heavy drinkers [5–7].
A recent study in a high cardiovascular risk model of chronic ischaemia in swine demonstrated that while myocardial perfusion was improved with both red wine and vodka consumption, it was only in animals supplemented with vodka that a lack of pericardial adhesions was encountered at reoperative sternotomy [8, 9]. The initial study, though demonstrating not only significantly fewer pericardial adhesions, but also significantly less intramyocardial fibrosis and transmural collagen deposition in the vodka-supplemented animals, failed to define the molecular mechanism by which the decrease in adhesions and fibrosis was achieved. Only the remote, normal ventricle was examined in the chronic ischaemia study, which is anatomically distant from the initial operative site where adhesions were the most dense. For this reason, in the current study, we examine the myocardium in the at-risk territory which includes the epicardial surface exposed in the surgical field from the initial operation, as well as the epicardial–pericardial attachments. Furthermore, we analysed two solubility fractions of the myocardial lysates. In addition to analysing protein expression in the radioimmunoprecipitation assay (RIPA) buffer-soluble fraction, we also analysed the myocardial lysate fraction soluble in a strong sodium dodecyl sulphate (SDS)-based detergent in order to solubilize proteins involved in epicardial–extracellular matrix (ECM) interactions, including proteins with a transmembrane domain.
This porcine model of chronic ischaemia with the concomitant risk factor modification by the administration of a high-fat/cholesterol diet was chosen to better replicate the human disease condition that it intends to model. Previous animal studies of induced chronic cardiac ischaemia in otherwise healthy animals have proven to be inadequate simulators of patients with coronary artery disease (CAD) who have ischaemia in the setting of multiple comorbidities that induce a chronic inflammatory response and endothelial dysfunction [10]. In fact, a high-fat/cholesterol diet has been shown to increase oxidative stress, inflammation and endothelial function in this porcine model [10, 11].
The current study uses this same high-fat/cholesterol-fed animal model with alcohol supplementation with an in-depth analysis of myocardial protein expression at the time of reoperative sternotomy with a focus on proteins involved in focal adhesion formation, cell–ECM interactions and the caspase proteases [12]. The caspase cascade system plays an integral role in the directed induction and execution of apoptosis and mediation of inflammation, cytokine maturation, proteolysis and protein–protein interactions [13].
MATERIALS AND METHODS
Animal model
Starting at 4 weeks of age, 28 male Yorkshire miniswine (Parson's Research, Amherst, MA, USA) were fed a 500 g/day of a high-fat/cholesterol diet throughout the 11-week experiment to simulate conditions of CAD. The hypercholesterolemic diet consisted of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate and 75% regular chow (Sinclair Research, Columbia, MO, USA). All animals had unlimited access to drinking water. After 4 weeks of diet modification, all animals underwent ameroid constrictor placement (Research Instruments SW, Escondito, CA, USA) to induce chronic cardiac ischaemia. For all surgical procedures, anaesthesia was induced with intramuscular injection of 5 mg/kg tiletamine HCl (Telazol, Fort Dodge Animal Health, NY, USA). Animals were endotracheally intubated and mechanically ventilated at 12–20 breaths/min. Anaesthesia was maintained with a gas mixture of 1.5–2.0 l/min of O2 and 0.75–3.0% isofluorane. Perioperative antibiotic prophylaxis for the ameroid constrictor placement consisted of a single dose of enrofloxacin (5 mg/kg i.v.) given after the induction of anaesthesia and continued (enrofloxacin 68 mg orally (PO) daily) PO daily) for 5 days postoperatively. Surgical approach for the ameroid constrictor placement was via a mini-left thoracotomy, and a titanium ameroid constrictor (1.75–2.25 mm internal diameter) was placed around the proximal left circumflex coronary artery (LCx) just distal to its take-off from the left main coronary artery. During a 2-min temporary occlusion of the LCx, 1.5 × 107 (5 ml) isotope-labelled gold microspheres (non-radioactive) were injected into the left atrium over a period of 30 s to determine the myocardial territory at risk through shadow labelling after cardiac harvest (BioPhysics Assay Laboratory, Worcester, MA, USA). The pericardium was reapproximated with three interrupted 4-0 Nurolon sutures (Ethicon, Somerville, NJ, USA). Postoperative pain was controlled with buprenorphine HCl intramuscular injection (0.03 mg/kg) at the end of the first operation and the placement of a transdermal fentanyl patch (4 μg/kg) continued for 72 h postoperatively. All animals received perioperative aspirin at 325 mg/day for prophylaxis against thrombo-embolic events starting 1 day before the first operation and continuing for a total of 5 days.
Postoperatively, animals were divided into three groups according to diet supplementation. The high-cholesterol control group (HCC, n = 9) continued the high-fat/cholesterol diet for the remaining 7 weeks of the experiment. High-cholesterol wine pigs were supplemented with 375 ml of red wine (2009 Pinot Noir, Black Mountain Vineyard, Napa and Sonoma, CA, USA) daily (12.5% EtOH/V, HCW, n = 9). High-cholesterol vodka pigs were supplemented with 112 ml of vodka (Rubinoff Vodka, Somerville, MA, USA) daily (40% EtOH/V, HCV, n = 9).
Seven weeks after initial operation, at 15 weeks of age, all animals underwent a final non-survival operation via a median sternotomy. Cardiac harvest included the collection of tissue from the collateral-dependent area at risk (AAR), in the distribution of the occluded LCx, as determined by shadow labelling of injected microspheres. Myocardial samples were snap-frozen in liquid nitrogen at the time of harvest.
All experiments were approved by the Rhode Island Hospital Institutional Animal Care and Use Committees. Animals were cared for in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals [14]’.
Myocardial lysate preparation
Myocardial lysis buffer was prepared by adding one protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN, USA), 250 µl each of phosphatase inhibitors two and three cocktails (Sigma-Aldrich, St Louis, MO, USA), and 320 µl of 2 M sodium fluoride to 25 ml of RIPA buffer (Boston BioProducts, Ashland, MA, USA) at 4°C. The RIPA-soluble myocardial lysate fraction was then prepared by homogenizing 150 mg of frozen (−80°C) myocardial tissue from the AAR in 1.0 ml of the above prepared RIPA buffer at 4°C using 6 × 2.0 mm diameter zirconium-oxide beads (Next Advance, Inc. Averill Park, NY, USA) in the Bullet Blender Blue tissue homogenizer (Next Advance, Inc.) for two, 5-min cycles. Lysates were then incubated at 4°C for 30 min and centrifuged at 14 000 × g for 10 min at 4°C. The supernatant was designated as the RIPA-soluble fraction. Prepared lysates were stored in liquid nitrogen.
The SDS-soluble lysate fraction was prepared by resolubilizing the pellet remaining from the centrifugation of the RIPA-soluble lysate as prepared above (pellet contains zirconium-oxide beads) in 1.0 ml of 2% SDS, 151 mM Tris–Base, 106 mM Tris–HCl and 0.51 mM ethylenediaminetetraacetic acid. Protease inhibitors, phosphatase inhibitors and sodium fluoride were added at the same concentration as above. The pellet and SDS buffer were then homogenized in the Bullet Blender for two, 5-min cycles, heated for 3 min at 100°C, centrifuged at 10 000 × g for 5 min, and the supernatant stored in liquid nitrogen designated as the insoluble lysate fraction. Total protein concentrations for all lysates were determined by Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) and Synergy Mx multi-mode microplate reader 7191000 (BioTek Instruments, Inc., Winooski, VT, USA).
Protein expression
Sixty μg of total protein from the SDS-soluble myocardial lysate fraction made from the AAR was fractionated by SDS–polyacrylamide gel electrophoresis using the NuPage Novex Bis-Tris Mini Gel system (Invitrogen, Carlsbad, CA, USA) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated at 4°C overnight with antibodies against focal adhesion kinase (FAK; Cell Signaling, Danvers, MA, USA), protein tyrosine kinase (also known as related adhesion focal tyrosine kinase, RAFTK; Cell Signaling), integrin α-5 (Int α5; Cell Signaling), integrin β-1 (Int β1; Cell Signaling), paxillin (Cell Signaling), vinculin (Cell Signaling), protein kinase C epsilon (PKCϵ; Cell Signaling) and phosphorylated PKCϵ (p-PKCϵ; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at dilutions recommended by the manufacturer. Both SDS-soluble and RIPA-soluble myocardial lysate fractions from the AAR were incubated at 4°C overnight with antibodies against caspase 3 (Cell Signaling), cleaved caspase 3 (Cell Signaling), caspase 9 (Cell Signaling), phosphorylated caspase 9 (p-Caspase 9; Cell Signaling) and caspase 12 (Cell Signaling) at dilutions recommended by the manufacturer followed by the appropriate horseradish peroxidase-linked secondary antibodies (1:4000, Jackson Immunoresearch, West Grove, PA, USA). Immune complexes were visualized via electrochemiluminescence (ECL) and photographed using GeneSnap software (Syngene, Cambridge, UK). Densiometry of the ECL signal was performed using ImageJ software (NIH, Bethesda, MD, USA). To ensure and correct for equal protein loading, membranes were probed with glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling), a constitutively expressed housekeeping protein. Raw data collected as arbitrary light units from ECL fluorescence and ImageJ densitometry were averaged for each group and expressed in fold change (FC) when compared with the HCC mean using Microsoft Excel Software (Microsoft, Redmond, WA, USA). Researchers were blinded to the study group during analysis of western blot data.
Statistical analysis
All results were reported as the mean ± standard error of the mean. Western blot expression data were analysed by the one-way analysis of variance (ANOVA) among groups followed by a post hoc Bonferroni test using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
SDS-soluble lysate fraction
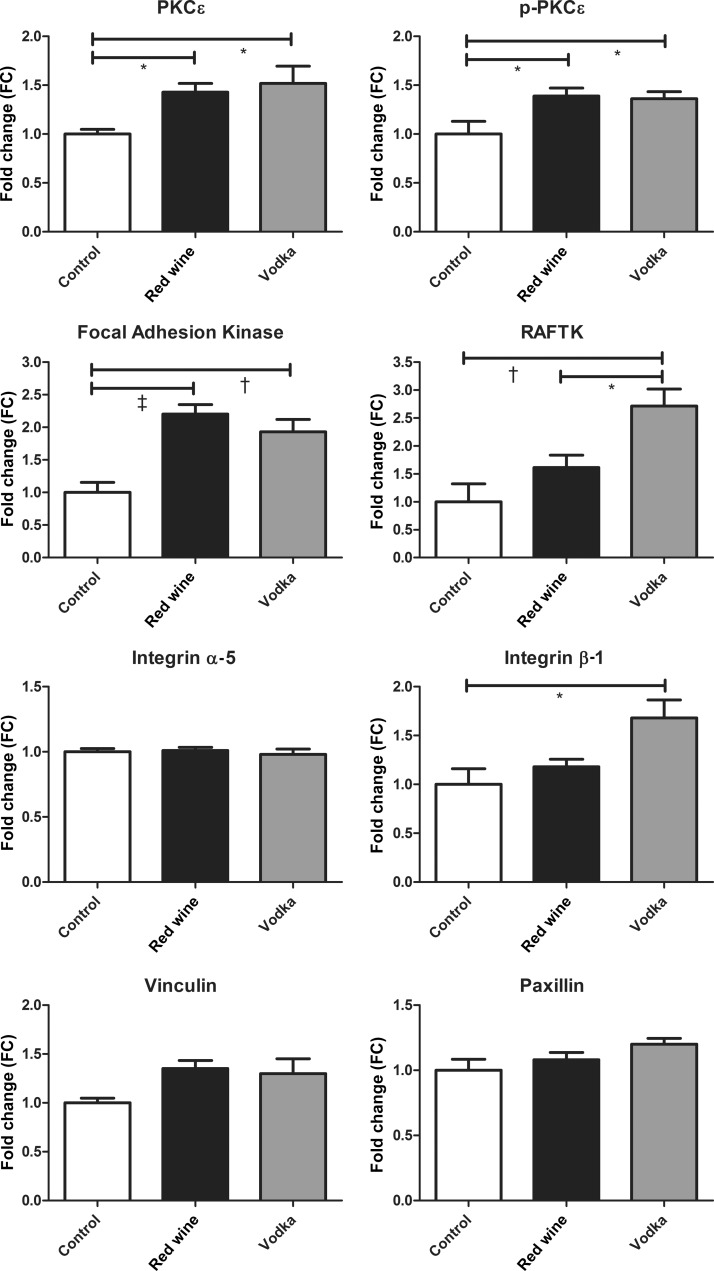
The expression of PKCϵ in the SDS-soluble myocardial lysate fraction was significantly higher in both treatment groups (high-cholesterol diet with red wine (HCW), and high-cholesterol diet with vodka (HCV)) compared with controls (high-cholesterol diet controls (HCC), ANOVA, P = 0.010), as was the expression of activated p-PKCϵ (ANOVA, P = 0.017). FAK was also higher in both treatment groups (HCW and HCV) compared with controls (ANOVA, P = 0.0001), whereas RAFTK expression was significantly higher in the HCV group compared with both HCW and controls (ANOVA, P = 0.001). Though there were no differences in the expression of Int α5 (ANOVA, P = 0.794), vinculin (ANOVA, P = 0.073) or paxillin (ANOVA, P = 0.096) between groups, the expression of Int β1 was significantly higher in the HCV group (ANOVA, P = 0.011). See Figure 1 for results of Bonferroni post-test P-values between groups.
Figure 1:
SDS-soluble fraction of the AAR. The expression of PKCϵ and proteins involved in focal adhesion formation in the SDS-soluble myocardial lysate fraction between the control group and animals supplemented with either red wine or vodka. Results are expressed as FC in expression when compared with controls. Bonferroni post-test P-values depicted as: *P < 0.05, †P < 0.01 and ‡P < 0.001. See Results for the report of one-way ANOVA P-values.
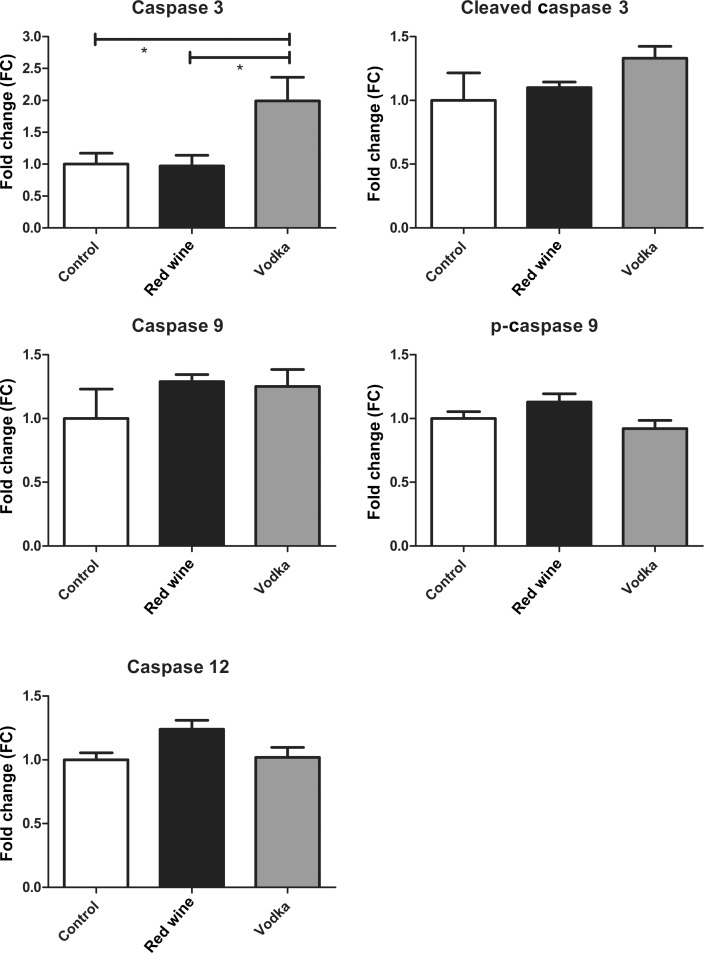
The expression of caspase 3 in the SDS-soluble myocardial lysate fraction was significantly higher in the HCV group compared with either HCW or controls (ANOVA, P = 0.018), whereas there were no differences in the expression of cleaved caspase 3 (ANOVA, P = 0.219), caspase 9 (ANOVA P = 0.390) or p-caspase 9 (ANOVA, P = 0.071). The expression of caspase 12 was different among groups (ANOVA, P = 0.043); however, the Bonferroni post-test did not reveal any differences between groups. See Figure 2 for results of Bonferroni post-test P-values between groups.
Figure 2:
SDS-soluble fraction of the AAR. Caspase protein expression in the insoluble myocardial lysate fraction between the control group and animals supplemented with either red wine or vodka. Results are expressed as FC in expression when compared with controls. Bonferroni post-test P-values depicted as: *P < 0.05. See Results for the report of one-way ANOVA P-values.
RIPA-soluble lysate fraction
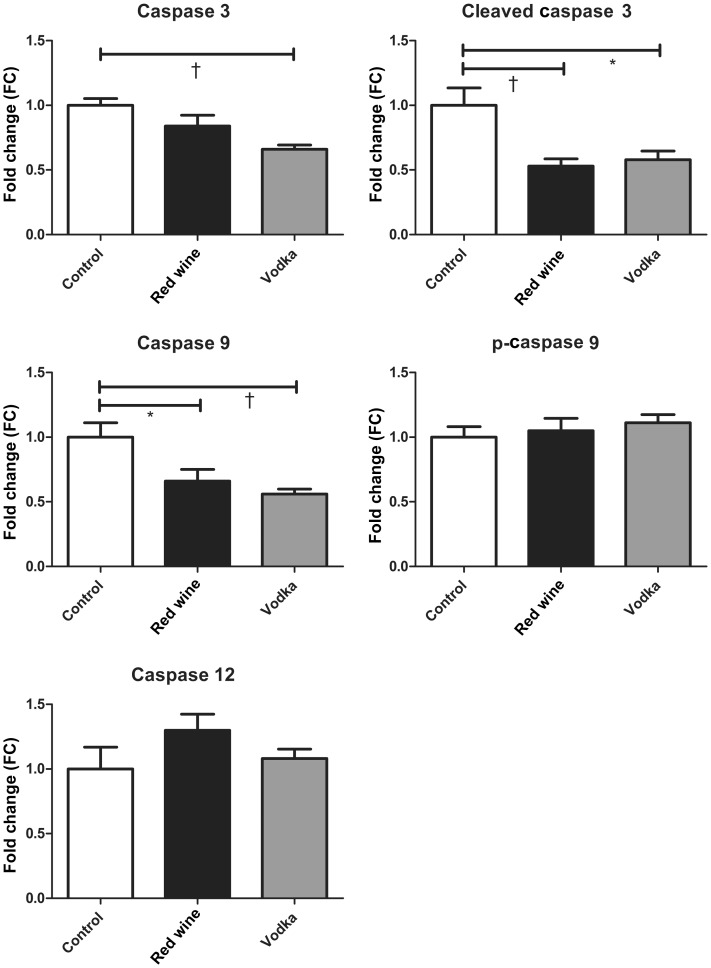
The expression of caspase 3 in the RIPA-soluble myocardial lysate fraction was lowest in the HCV group, but also decreased in the HCW group compared with controls (ANOVA, P = 0.002). Cleaved caspase 3 expression was also lower in both treatment groups (HCW and HCV) compared with controls (ANOVA, P = 0.003). Caspase 9 expression followed the same trend (ANOVA, P = 0.003), whereas there were no differences in the expression of p-caspase 9 or caspase 12 among groups (ANOVA, P = 0.624 and 0.226, respectively). See Figure 3 for results of Bonferroni post-test P-values between groups.
Figure 3:
RIPA-soluble fraction of the AAR. Caspase protein expression in the RIPA-soluble myocardial lysate fraction between the control group and animals supplemented with either red wine or vodka. Results are expressed as FC in expression when compared with controls. Bonferroni post-test P-values depicted as: *P < 0.05 and †P < 0.01. See Results for the report of one-way ANOVA P-values.
DISCUSSION
Both total and p-PKCϵ expression were increased in the SDS-soluble fraction of the chronically ischaemic myocardium in both the red wine and vodka-supplemented groups compared with controls. This is likely a direct effect of ethanol, as both treatment groups were receiving an identical amount of ethanol in their diets, and the expression of PKCϵ was not different between the two treatment groups. PKCϵ has been implicated in coronary graft and myocardial protection after ischaemic injury by protecting mitochondria as well as playing a regulatory role in apoptotic signalling and has been shown to play a central role in cardioprotection, specifically by blunting pathophysiological responses to chronic myocardial hypoxia [15–17]. This may explain why previous studies in this animal model demonstrated significantly improved myocardial perfusion in the red wine and vodka treatment groups when compared with controls [8].
Though the up-regulation of FAK in both the ethanol-treated groups (red wine and vodka) and of its structural relative, RAFTK, only in the vodka-supplemented group may seem paradoxical, as there was a reduction in adhesions in the vodka treated group, the role of RAFTK in the caspase cascade may offer some insight into this finding. Cardiac remodelling, as it is related to cardiomyocyte apoptosis, and cellular and cytoskeletal focal adhesion formation are regulated by RAFTK. This has been demonstrated specifically though the interaction of RAFTK and caspase 3 activation [18]. Thus, the increase in caspase 3 expression observed only in the vodka-supplemented group in the SDS-soluble myocardial fraction may explain why the increase in FAK and RAFTK expression was correlated with a decrease in adhesion formation. Caspase 3 activity in the myocardium has been directly associated with apoptosis, especially in the setting of hypoxia and diabetes [19–22]. The increased expression of Int β1 seen only in the vodka-treated group could be the sequelae of a lack of negative feedback from adhesion formation, as integrin-mediated kinase expression has been directly correlated with cell survival, fibrosis and cell adhesion in ischaemic myocardium [23]. Likewise, the seemingly paradoxical increase in FAK and RAFTK in the vodka-treated group may also be the sequelae of a lack of negative feedback from adhesion formation.
In the RIPA-soluble fraction of the AAR, caspase expression was decreased in both the alcohol-supplemented groups (caspase 3, cleaved caspase 3 and caspase 9). Here, this down-regulation of proapoptotic caspases appears to be an effect of ethanol in general as it is seen in both the red wine and vodka-treated groups and not specific to vodka alone [13]. This decreased apoptotic regulation in the ischaemic myocardium in animals supplemented with ethanol corroborates the well-established cardioprotective role of moderate alcohol consumption and supports the overt improvements in myocardial perfusion seen in this animal model with the administration of ethanol [3].
The up-regulation of RAFTK expression in the SDS-soluble fraction and concomitant increased expression of the pro-apoptotic caspase 3 seen only with the supplementation of ethanol in the form of vodka offers mechanistic insight into why a lack of pericardial adhesions was observed in this group at reoperation. The specificity of this RAFTK and caspase 3 overexpression to the SDS-soluble lysate fraction and not to the RIPA-soluble myocardial lysate offers an explanation as to why there is no evidence for increased myocardial death in the vodka-treated animals and no obvious negative impact on myocardial function. The fact that focal adhesions are largely an interaction of the cell to the ECM and that these proteins are at least in part, transmembrane, further supports this proposed mechanism of RAFTK and caspase 3 up-regulation in the SDS-soluble, or transmembrane, cardiac tissue areas of cell–ECM interaction [12].
CONCLUSIONS
The findings presented in this manuscript not only provide insight into a proposed molecular mechanism by which the administration of vodka inhibits the formation of postoperative pericardial adhesions, but also offers an explanation as to why these proapoptotic effects are specific to the pericardial tissues and do not increase cell death in ischaemic, surrounding myocardium. Furthermore, these findings emphasize the need for further investigation into the potential use of vodka or ethanol administration in the postoperative period as a systemic treatment for the prevention of pericardial adhesions. Ethanol is currently a commercially available pharmaceutical agent that is widely consumed by humans at doses consistent with this current animal study. As many cardioprotective effects of ethanol consumption at moderate doses are increasingly supported in medical literature, this study is pertinent to current patient management. A large number of adult cardiac surgery patients consume alcohol regularly. Thus, although we certainly would not advocate for the current non-drinking patients to begin drinking alcohol, as these results are preliminary, further discussion is warranted in asking whether current, moderate drinkers should abstain.
LIMITATIONS
The most obvious limitation to any animal study lies in its translatability to human physiology. Though porcine cardiac disease and physiology does seem to be a very good large-animal model for human disease, there are likely some aspects which are unique to swine. Another important limitation that warrants addressing is the use of the at-risk myocardium in this study, introducing ischaemia as an independent variable which alone may be contributing to some of the observed differences in myocardial protein expression between the initial study which examined the remote territory of the myocardium and the current study. However, in the current study, both the treatment groups as well as the controls were chronically ischaemic; thus, this should not be a confounder between groups. In order to remove chronic ishcemia as a confounder, a follow-up study with surgical exposure of the myocardium and disruption of the pericardium, but without placement of an ameroid constrictor, would be indicated at this time. However, such a study in which the variable of chronic myocardial ishcaemia has been removed may not be as good of a model for cardiac patients with CAD undergoing redo coronary artery bypass grafting as a model which includes ameroid-induced ischaemia, because many reoperative cardiac patients do suffer from chronic myocardial ischaemia.
Funding
This work was supported by grants from the National Institute of Health (R01HL46716, R01HL69024 and R01HL85647 to Frank W. Sellke); the National Institute of Health Training Grants (5T32-HL076134 to Antonio D. Lassaletta and 5T32-HL094300 to Louis M. Chu and Nassrene Y. Elmadhun) and by the Thoracic Surgery Foundation for Research and Education Fellowship to Antonio D. Lassaletta
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We would like to thank the animal facility staff at the Rhode Island Hospital.
REFERENCES
- 1.Kaushal S, Patel SK, Goh SK, Sood A, Walker BL, Backer CL. A novel combination of bioresorbable polymeric film and expanded polytetrafluoroethylene provides a protective barrier and reduces adhesions. J Thorac Cardiovasc Surg. 2011;141:789–95. doi: 10.1016/j.jtcvs.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Lopes JB, Dallan LA, Moreira LF, Campana Filho SP, Gutierrez PS, Lisboa LA, et al. Synergism between keratinocyte growth factor and carboxymethyl chitosan reduces pericardial adhesions. Ann Thorac Surg. 2010;90:566–72. doi: 10.1016/j.athoracsur.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 3.Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, et al. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact CardioVasc Thorac Surg. 2010;10:213–6. doi: 10.1510/icvts.2009.215251. [DOI] [PubMed] [Google Scholar]
- 4.Meisel JA, Fallon EM, Le HD, Nehra D, de Meijer VE, Rodig SJ, et al. Sunitinib inhibits postoperative adhesions in a rabbit model. Surgery. 2011;150:32–8. doi: 10.1016/j.surg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Foerster M, Marques-Vidal P, Gmel G, Daeppen JB, Cornuz J, Hayoz D, et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol. 2009;103:361–8. doi: 10.1016/j.amjcard.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 6.Hansel B, Thomas F, Pannier B, Bean K, Kontush A, Chapman MJ, et al. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the Urban Paris-Ile-de-France Cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr. 2010;64:561–8. doi: 10.1038/ejcn.2010.61. [DOI] [PubMed] [Google Scholar]
- 7.Miyamae M, Kaneda K, Domae N, Figueredo VM. Cardioprotection by regular ethanol consumption: potential mechanisms and clinical application. Curr Drug Abuse Rev. 2010;3:39–48. doi: 10.2174/1874473711003010039. [DOI] [PubMed] [Google Scholar]
- 8.Chu L, Lassaletta A, Robich M, Yuhong L, Burgess T, Laham R, et al. Effects of red wine and vodka on collateral-dependent perfusion and cardiovascular function in hypercholesterolemic swine. Circulation. 2012;126:S65–72. doi: 10.1161/CIRCULATIONAHA.111.082172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassaletta AD, Chu LM, Sellke FW. Effects of alcohol on pericardial adhesion formation in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 2012;143:953–9. doi: 10.1016/j.jtcvs.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 11.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–41. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 12.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–27. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special report: the 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–8. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Dong S, Teng Z, Lu FH, Zhao YJ, Li H, Ren H, et al. Post-conditioning protects cardiomyocytes from apoptosis via PKC(epsilon)-interacting with calcium-sensing receptors to inhibit endo(sarco)plasmic reticulum-mitochondria crosstalk. Mol Cell Biochem. 2010;341:195–206. doi: 10.1007/s11010-010-0450-5. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T, et al. Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation. 2004;110:II194–9. doi: 10.1161/01.CIR.0000138389.22905.62. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy J, Lochner A, Opie LH, Sack MN, Essop MF. PKCepsilon promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3beta inhibition. J Cell Physiol. 2011;226:2457–68. doi: 10.1002/jcp.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melendez J, Turner C, Avraham H, Steinberg SF, Schaefer E, Sussman MA. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J Biol Chem. 2004;279:53516–23. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- 19.Aljinovic J, Vukojevic K, Kosta V, Guic MM, Saraga-Babic M, Grkovic I. Histological differences in healing following experimental transmural infarction in rats. Histol Histopathol. 2010;25:1507–17. doi: 10.14670/HH-25.1507. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Yong SL, Drinko JK, Popovic ZB, Shryock JC, Belardinelli L, et al. LQTS mutation N1325S in cardiac sodium channel gene SCN5A causes cardiomyocyte apoptosis, cardiac fibrosis and contractile dysfunction in mice. Int J Cardiol. 2011;147:239–45. doi: 10.1016/j.ijcard.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adderley SR, Fitzgerald DJ. Glycoprotein IIb/IIIa antagonists induce apoptosis in rat cardiomyocytes by caspase-3 activation. J Biol Chem. 2000;275:5760–6. doi: 10.1074/jbc.275.8.5760. [DOI] [PubMed] [Google Scholar]
- 22.Chen JX, Zeng H, Reese J, Aschner JL, Meyrick B. Overexpression of angiopoietin-2 impairs myocardial angiogenesis and exacerbates cardiac fibrosis in the diabetic db/db mouse model. Am J Physiol Heart Circ Physiol. 2012;302:H1003–12. doi: 10.1152/ajpheart.00866.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song SW, Chang W, Song BW, Song H, Lim S, Kim HJ, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27:1358–65. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]