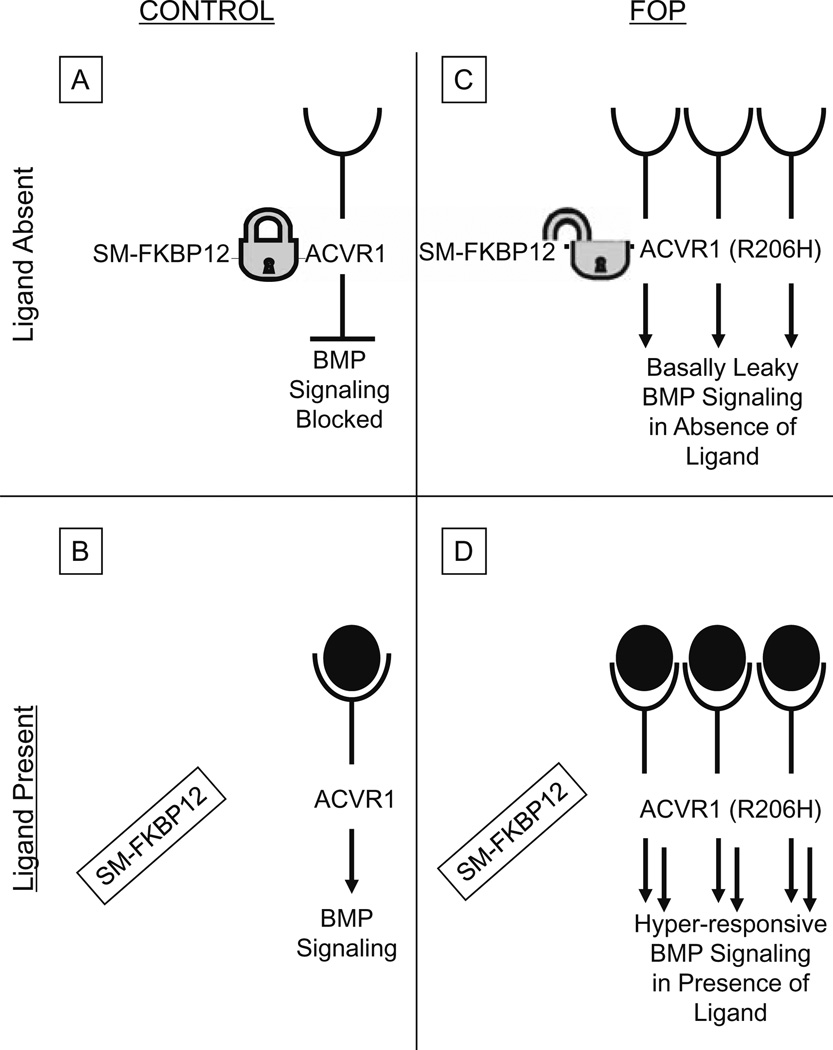
Fig. 2.
Hypothetical schema of bone morphogenetic protein (BMP) signaling in FOP cells. [Adapted from Kaplan FS, et al (2008) Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol, in press.] In control cells (A), in the absence of ligand, the Smad/Smurf-FKBP12 (SM-FKBP12) complex binds ACVR1 (a BMP type I receptor) and prevents its promiscuous phosphorylation by the constitutively active type II BMP receptor (not shown). SM-FKBP12 also promotes ubiquitin-associated degradation of ACVR1 in the absence of ligand, thus maintaining low steady-state levels of ACVR1 at the cell membrane. Following ligand binding in control cells (B), SM-FKBP12 inhibition is released, thus allowing the constitutively active BMP type II receptor (not shown) to phosphorylate ACVR1 and promote SMAD1, −5, −8 phosphorylation and downstream BMP signaling. In FOP cells, SM-FKBP12 does not bind appropriately to the mutant receptor [ACVR1 (R206)]. Thus, inhibition of BMP signaling is impaired in the absence of ligand, and basal leakiness of BMP signaling occurs (C). Additionally, because the SM-FKBP12 complex cannot properly target the mutant ACVR1 (R206H) receptor for ubiquitin-associated degradation, ACVR1 might be expected to accumulate at the cell surface. Thus, in the presence of ligand (D), hyper-responsive BMP signaling might be predicted to occur. SM-FKBP12, Smad/Smurf-FKBP12 complex; arrows, signaling promoted; (by FOP mutation); open cups, extracellular ligand-binding domain of ACVR1; filled-in circles, BMP; filled-in circles inside of open cups, BMP ligand binding to ACVR1